 PDF(16902 KB)
PDF(16902 KB)


 PDF(16902 KB)
PDF(16902 KB)
 PDF(16902 KB)
PDF(16902 KB)
普鲁士蓝基钠离子电池正极材料的改性
Modification of Cathode Materials for Prussian Blue-Based Sodium-Ion Batteries
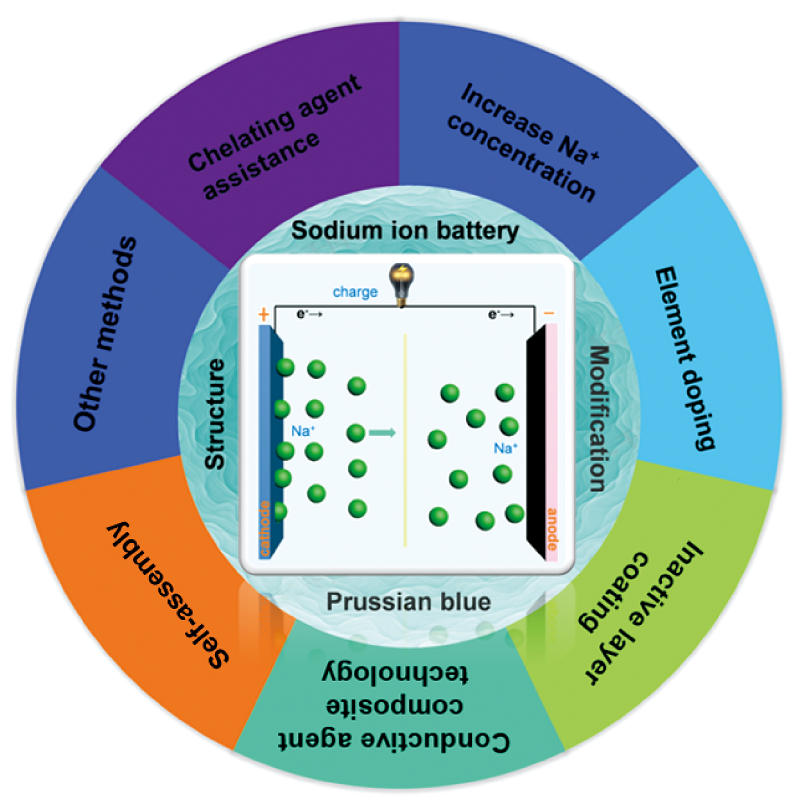
普鲁士蓝(PB)及其类似物(PBAs)由三维框架结构构成,能够为钠离子的嵌入脱出提供较宽的通道,是一种理想的钠离子电池(SIB)正极材料。然而,PBAs材料中存在大量水分子和空位,在很大程度上降低了钠离子的存储位点,且金属有机框架中的过渡金属离子易于在循环过程中析出,导致PBAs正极材料的储钠容量有限和循环稳定性不佳。近年来,多种PBAs改性技术被研究出来,使其电化学储钠性能得到明显提升。本文基于近期相关工作和已有的文献报道,从不同改性技术的工艺设计、制备方法、电化学行为等方面进行了总结,系统综述并展望了PBAs正极材料各种改性技术在钠离子电池中的研究进程。
Prussian blue (PB) and its analogues (PBAs), which are composed of three-dimensional frame structure, are ideal cathode materials for sodium ion battery (SIB) and can provide a wide channel for sodium ion embedding and removal. However, there are a lot of water molecules and vacancies in PBAs materials, which greatly reduces the storage sites of sodium ions. Furthermore, transition metal ions in the metal organic framework are easy to dissolve during the cycles, resulting in limited sodium storage capacity and poor cycle stability of PBAs cathode materials. In recent years, a variety of PBAs modification technologies have been developed to improve their sodium storage performance. Based on recent related work and existing literature reports, this paper summarizes the process design, preparation methods, electrochemical behavior and other aspects of different modification technologies, and systematically reviews and prospects the research progress of various modification technologies of PBAs cathode materials in sodium ion batteries.
1 Introduction
2 Structure of Prussian blue and its analogues
3 Modification method of Prussian blue cathode material
3.1 Chelating agent assisted method
3.2 Increase Na+concentration
3.3 Element doping
3.4 Inactive layer coating
3.5 Conductive agent composite technology
3.6 Self-assembly
3.7 Other modification methods
4 Conclusion and outlook
钠离子电池 / 普鲁士蓝 / 正极材料 / 晶体结构 / 反应机理 / 改性方法
sodium ion battery / prussian blue / cathode material / crystal structure / reaction mechanism / modification method
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
/
| 〈 |
|
〉 |