 PDF(35978 KB)
PDF(35978 KB)


 PDF(35978 KB)
PDF(35978 KB)
 PDF(35978 KB)
PDF(35978 KB)
甲醇制烯烃反应中的凝聚态化学
Methanol to Olefins (MTO): A Condensed Matter Chemistry
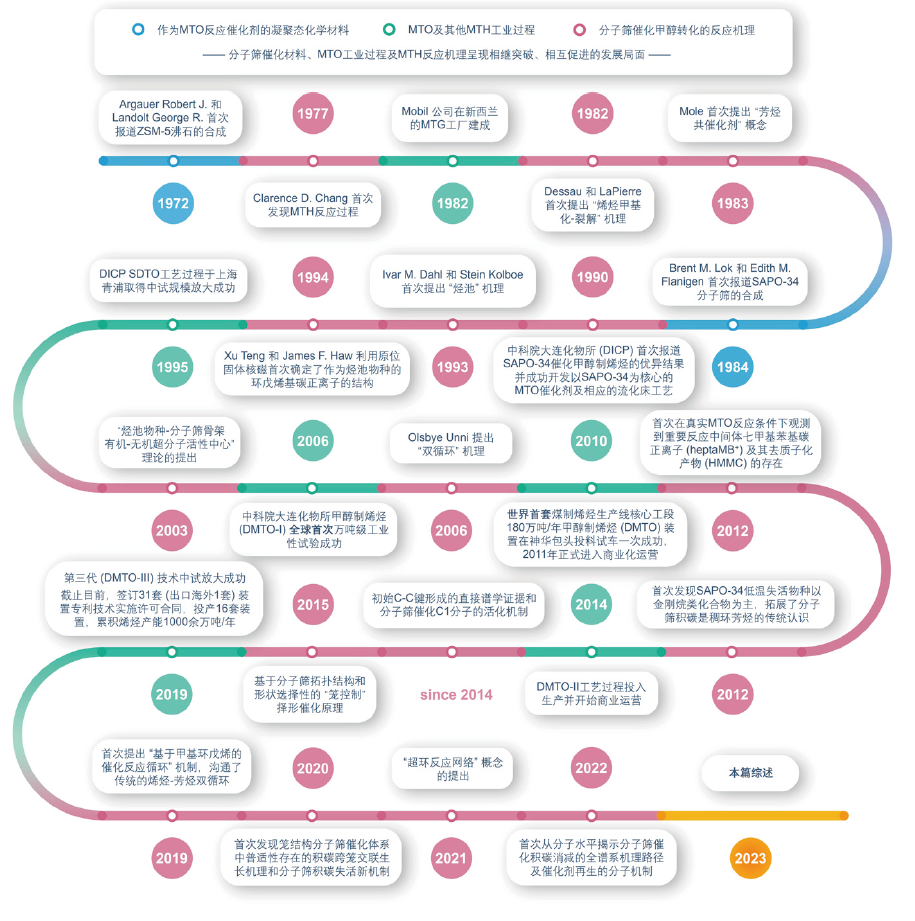
催化技术在现代工业生产和日常生活中发挥着举足轻重的作用,也是凝聚态化学材料和应用的重要内容。甲醇制烯烃反应在凝聚态晶体多孔材料上实现,是非石油资源制取低碳烯烃的重要途径,也是凝聚态材料催化应用的典型案例。反应机理和分子筛积碳机制是多相催化领域重要的研究方向。甲醇制烯烃反应是一个动态化学过程,经历诱导期、高效反应期、失活期和催化剂再生,分子筛纳米限域空间内活性有机物种和积碳物种的演变引导了这个催化反应历程。本文围绕这一主题,分别介绍了甲醇制烯烃反应分子筛催化材料及基于主客体化学的结构组成-反应性能的构效关系、甲醇转化反应的分子活化机制、动态催化反应网络以及基于分子筛-积碳主客体相互作用发展的择形催化原理和分子筛积碳失活机理及消碳再生机制。希望通过本文加深对分子筛催化甲醇制烯烃反应中的凝聚态化学的认识,并期待以凝聚态化学为指导,进一步推动分子筛催化材料和催化过程的优化和发展,为今后高效催化剂及催化体系的开发提供指导。
Catalysis is an essential component of condensed matter chemistry, with broad applications in contemporary industrial manufacturing and daily life. Methanol-to-olefins (MTO) reaction, facilitated by condensed-matter porous materials, represents a significant catalytic pathway for the production of light olefins from non-petroleum sources, exemplifying heterogeneous catalytic applications. Investigating reaction mechanisms and catalyst coking/decoking mechanisms is a central focus in catalysis research. The MTO reaction, transpiring within the confined spaces of zeolites and/or molecular sieves, encompasses a dynamic chemical process comprising an induction period, a highly efficient stage, catalyst deactivation, and catalyst regeneration. The formation, evolution, and degradation of active organic species and coke species within the nano-confined spaces of zeolites guide the course of the catalytic reaction. This feature review primarily highlights zeolite/molecular sieve catalysts for the MTO reaction, elucidating the structural-reaction-deactivation relationship based on host-guest chemistry, activation mechanisms of C1 reactants, the catalytic reaction network governed by dynamic mechanisms, chemistries involved in zeolite coking and decoking behavior, as well as the mechanisms of catalyst deactivation and regeneration. The ultimate aim is to provide a profound understanding of condensed matter chemistry in the context of heterogeneous methanol-to-olefins chemistry, thus advancing zeolite catalysis theory and fostering the development of efficient MTO catalysts and high-efficiency, low-carbon catalytic processes under the guidance of condensed matter chemistry.
1 Introduction
2 Catalysts for methanol-to-olefins
2.1 ZSM-5 catalyst with MFI topology structure
2.2 SAPO-34 with CHA topology structure
2.3 Other catalysts with 8-MR pore opening and cavity structure
3 Catalytic reaction mechanism for methanol conversion
3.1 Direct mechanism
3.2 Indirect mechanism
4 Mechanisms of catalyst deactivation/regeneration by zeolite coking/decoking for methanol conversion
4.1 Deactivation mechanism and chemistry involved in zeolite coking
4.2 Regeneration mechanism and chemistry involved in zeolite decoking
5 Conclusions and outlook
凝聚态化学 / 甲醇制烯烃 / 分子筛 / 反应机理 / 催化剂失活 / 催化剂再生
condensed matter chemistry / methanol-to-olefins / molecular sieve / reaction mechanism / catalyst deactivation / catalyst regeneration
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
/
| 〈 |
|
〉 |