 PDF(28259 KB)
PDF(28259 KB)


Application of Polyacrylonitrile in the Electrolytes of Lithium Metal Battery
Yu Xiaoyan, Li Meng, Wei Lei, Qiu Jingyi, Cao Gaoping, Wen Yuehua
Prog Chem ›› 2023, Vol. 35 ›› Issue (3) : 390-406.
 PDF(28259 KB)
PDF(28259 KB)
 PDF(28259 KB)
PDF(28259 KB)
Application of Polyacrylonitrile in the Electrolytes of Lithium Metal Battery
With the rapid development of portable electronic devices, electric vehicles, and smart grids, there is an increasing interest in high-energy-density lithium metal batteries. Uneven Li stripping or deposition on the surface of lithium metal will lead to the growth of lithium dendrites, which can easily pierce the separator and cause the short circuit in the battery. Moreover, the highly reactive lithium metal will continue to react with the electrolyte, resulting in an unstable solid electrolyte. interfacial (SEI) film and irreversible capacity loss. Taking high-energy-density and high safety into account is a key scientific problem that needs to be solved urgently in the development and application of lithium metal batteries. The interaction of strong electron withdrawing group (C≡N) in polyacrylonitrile (PAN) polymer and C=O in carbonate solvent can form a more stable SEI film. As a lithium anode coating, PAN can also inhibit the growth of lithium dendrites. In addition, due to the low lowest unoccupied molecular orbital, high electrochemical stability and wide electrochemical window, PAN can be regard as polymer electrolytes for lithium metal batteries, and matched with a high-voltage cathode to achieve both high energy density and safety. Thus, PAN polymer has significant potential application in electrolytes for lithium metal batteries. This review mainly starts from the different states of electrolytes (liquid, gel, and solid state). Recent research development of PAN polymer as separators and lithium anode protective layers in liquid electrolytes, as well as its application in gel electrolytes and solid-state electrolytes are presented. Finally, the review prospects the development trend of PAN polymer in lithium metal battery electrolytes.
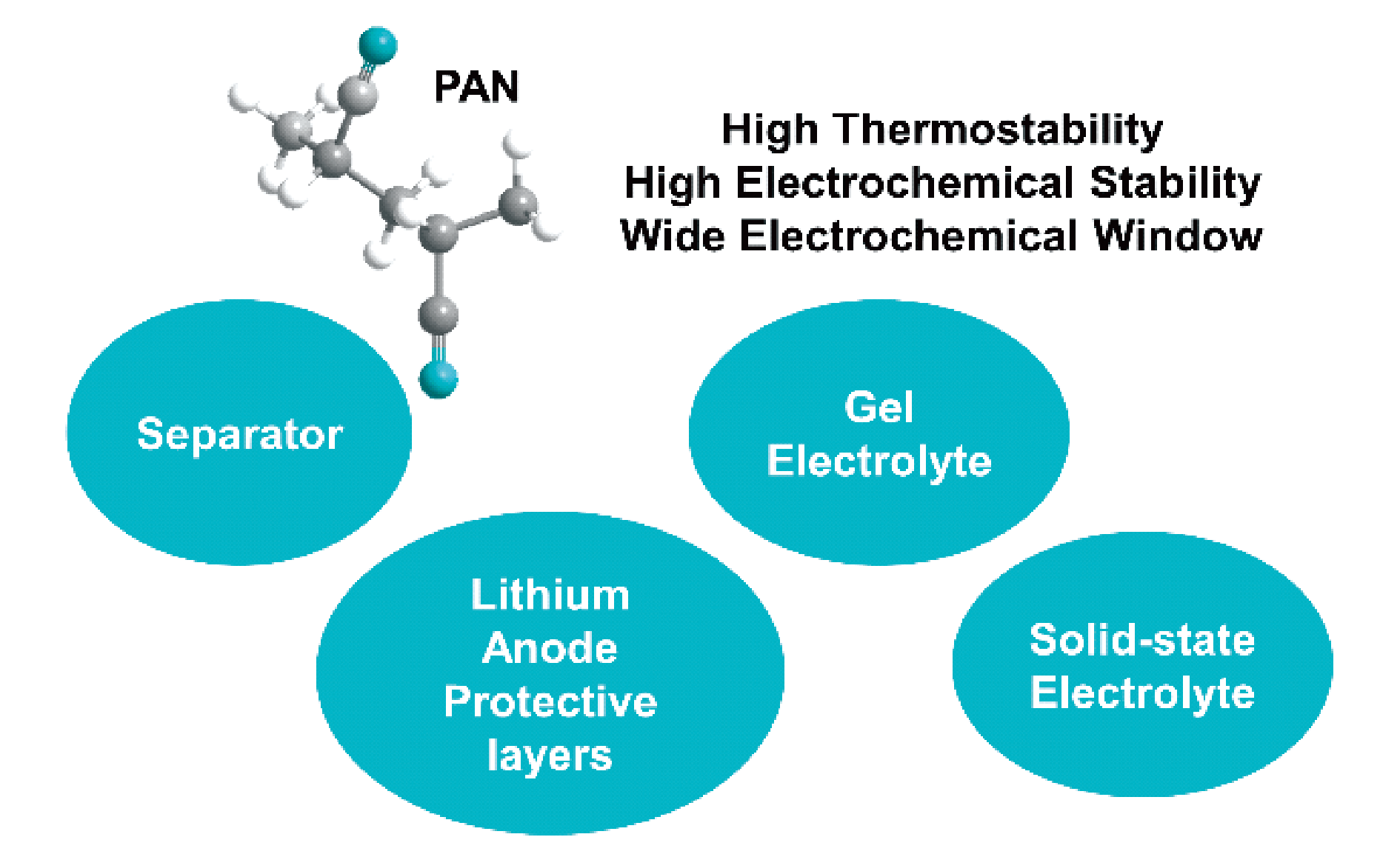
polyacrylonitrile / separator / lithium anode / gel electrolyte / solid-state electrolyte
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
(杨琪, 邓南平, 程博闻, 康卫民. 化学进展, 2021, 33(12): 2270.).
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
/
| 〈 |
|
〉 |