 PDF(13040 KB)
PDF(13040 KB)


Strigolactone and Its Novel Derivatives
Zhaoyong Kang, Xiaoqi Dong, Shengnan Liu, Qingzhi Gao
Prog Chem ›› 2023, Vol. 35 ›› Issue (9) : 1341-1356.
 PDF(13040 KB)
PDF(13040 KB)
 PDF(13040 KB)
PDF(13040 KB)
Strigolactone and Its Novel Derivatives
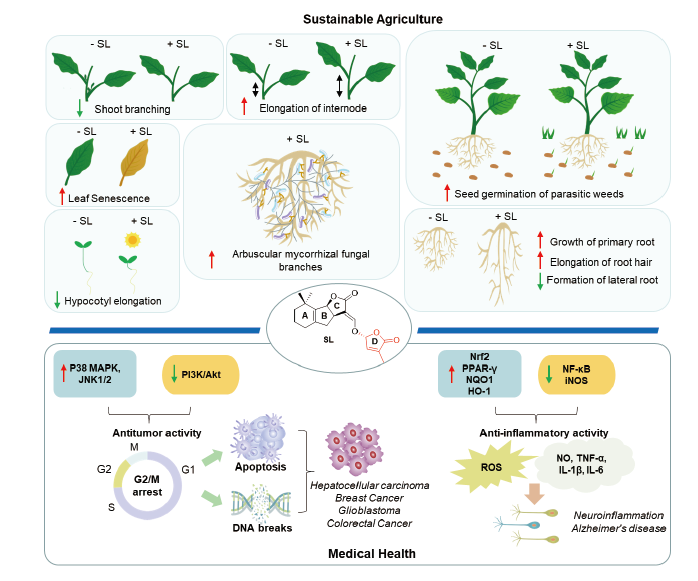
Strigolactones (SLs) are the most concerned endogenous sesquiterpenoid phytohormones.Recent studies have shown that strigolactones play crucial roles in inhibition of plant hypocotyl elongation and crop tillering, regulating root growth and development, stimulation of parasitic weed seed germination, coordinating the symbiotic interaction between parasitic plants and fungi, as well as regulation of plant response to biotic or abiotic stresses. Therefore, it is considered to be a new type of phytohormone with great development value and application potential in the field of agricultural science and plant protection. In addition, SL derivatives have also attracted much attention in the field of innovative drug research as the studies have found that: (1) SLs exhibit inhibitory activities against several tumor cell lines such as liver cancer, breast cancer, prostate cancer, glioblastoma, and colorectal cancers; (2) they possess anti-inflammation and glucose metabolism inhibitory activity. This paper aims to review the latest research progress of strigolactone and its structural derivatives with brief analysis on their biological activity, mechanism of action and structure-activity relationship. We hope this review provide guidance and directions on molecular design, development and utilization of SL natural products.
1 Introduction
2 Structural features and classification of strigolactones
3 The biosynthetic pathway and signal transduction mechanism of strigolactones
4 Structural characteristics and classification of natural strigolactones
5 Structural characteristics and classification of synthetic strigolactones
5.1 Canonical derivatives of strigolactone
5.2 Non-canonical derivatives of strigolactone
6 Conclusion and outlook
strigolactones / new phytohormone / green agriculture / biomedicine / molecular mechanism
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
/
| 〈 |
|
〉 |