 PDF(8456 KB)
PDF(8456 KB)


Design and Synthesis of Degradable Polyolefins
Huiping Yu, Yawei Qin, Jinyong Dong
Prog Chem ›› 2023, Vol. 35 ›› Issue (9) : 1294-1303.
 PDF(8456 KB)
PDF(8456 KB)
 PDF(8456 KB)
PDF(8456 KB)
Design and Synthesis of Degradable Polyolefins
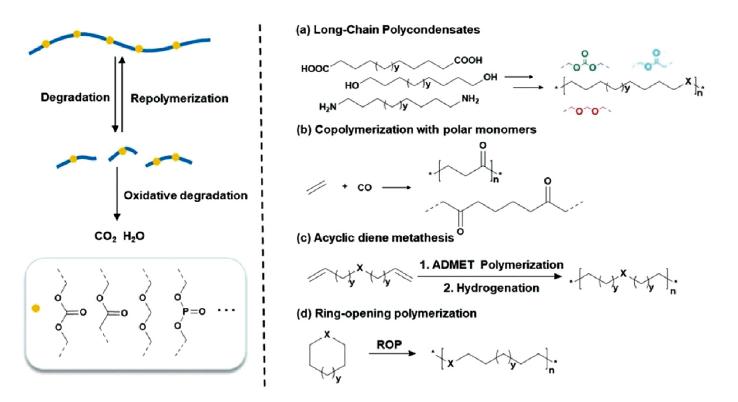
Polyolefin is thermoplastic universal plastic widely used in daily life. However, the overuse of polyolefin plastic and lack of degradability has led to a large amount of plastic waste, as well as growing land and marine pollution problems. The overwhelming majority of post-consumer polyolefin plastic is not recycled. Obstacles to the recycling of waste plastic include high energy consumption, low utilization rate of recycled products, low added value, and other wastes generated in the recycling process. Polyolefins degrade very slowly in the environment, and the addition of co-degraders can also cause environmental pollution. A feasible alternative is to redesign and synthesize degradable polyolefins, which can solve waste plastic problem from the source. The synthesis of degradable polyolefins has been extensively studied over the past half century. This paper summarizes the degradation mechanism of polyolefins, including oxidative degradation and co-degradation technology. Meanwhile we review four approaches to synthesizing degradable polyolefins, which cover condensation of long-chain bifunctional monomers, copolymerization with polar monomers, acyclic diene metathesis, and ring-opening polymerization. Among them, olefin metathesis polymerization has significantly expanded the types of degradable polyolefins due to the superior tolerance of the catalysts to functional groups, such as polyester, polyacetal, polycarbonate, polyphosphoester. We discuss the forward-looking synthetic approaches offered by current research and the challenges that these degradable materials face in truly replacing polyolefin materials. Finally, we propose our perspective on the opportunities and challenges in this field.
1 Introduction
2 Degradation mechanism of polyolefin
2.1 Oxidative degradation
2.2 Co-degradation technology
3 Synthesis of degradable polyolefins
3.1 Polycondensation of long chain difunctional monomers
3.2 Copolymerization with polar monomers
3.3 Acyclic diene metathesis
3.4 Ring-opening polymerization
4 Conclusion and outlook
polyolefin / degradable / condensation of long-chain bifunctional monomers / copolymerization with polar monomers / acyclic diene metathesis / ring-opening polymerization
| [1] |
|
| [2] |
|
| [3] |
(刘朝艳. 塑料工业, 2022, 50(04): 1.).
|
| [4] |
|
| [5] |
|
| [6] |
(刘雪辉, 徐世美, 张帆, 汪秀丽, 王玉忠. 高分子学报, 2022, 9: 1005.).
|
| [7] |
|
| [8] |
|
| [9] |
(骆希, 詹佳慧, 张士成. 能源环境保护, 2023, 37(1): 194.).
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
(宫涛. 精细与专用化学品, 2014, 22(6): 11.).
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
/
| 〈 |
|
〉 |