 PDF(5327 KB)
PDF(5327 KB)


Ball-Milled Click Chemistry: A Solvent-Free Green Chemistry
Xinqi Guan, Yuan Sang, Hailing Liu
Prog Chem ›› 2024, Vol. 36 ›› Issue (3) : 401-415.
 PDF(5327 KB)
PDF(5327 KB)
 PDF(5327 KB)
PDF(5327 KB)
Ball-Milled Click Chemistry: A Solvent-Free Green Chemistry
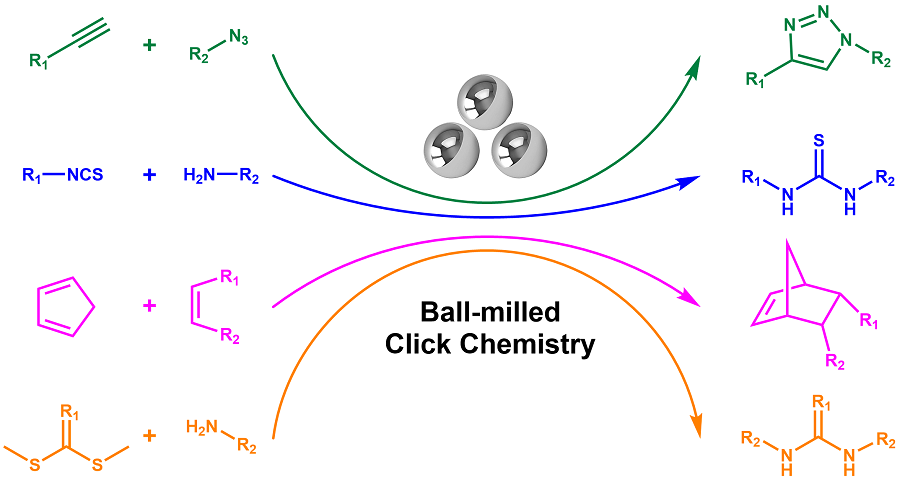
Click chemistry won the Noble Prize in 2022 due to easy synthesis, high selectivity, single product, and no toxic side product. Click chemistry was originally designed as green chemistry to work in aqueous solutions or environmentally friendly organic solvents. However, due to the poor solubility of reactants, polar and toxic solvents are usually required to use. The solvent used violates the concept of green chemistry, as well as increases the cost. These issues hinder click chemistry to be a state-of-art green chemistry. One of the solutions to optimize click chemistry is to avoid using any solvent. Herein, ball-milled mechanochemistry does not limit reactants’ solubility and could avoid solvent use. Ball-milled mechanochemistry is a new kind of chemical reaction that is conducted in a ball mill, is induced by mechanical force, and needs no solvent or a minimal amount of solvent. As a new way of organic synthesis, ball-milled mechanochemistry could easily achieve the low-energy carbon-heteroatom bonds, which constitute the linkages in click chemistry. Therefore, it could integrate with click chemistry and achieves ball-milled click chemistry. In comparison to traditional solution click chemistry, ball-milled click chemistry avoids solvent use. Moreover, it is even superior in the ways that the reaction time is shortened, the reaction temperature is lowered, and the catalyst used is simplified. In this review, ball-milled click chemistry examples are reported as much as the authors can find, including CuAAc, Diels-Alder, amine and isothiocyanate reactions, amine thiol reactions, and nitroxide radical coupling reactions. To provide readers with a better ball-milled click chemistry manual, this paper also contains ball mill machine choice guidance, liquid-assisted grinding choice guidance, and factors impacting ball-milled click chemistry conversion, including catalyst choice, additive choice, ball choice, stoichiometry, and milling time.
1 Introduction
1.1 Ball mill machines
1.2 Liquid/solid assisted grinding
2 Ball-milled click chemistry
2.1 Ball-milled CuAAc
2.2 Ball-milled Diels-Alder
2.3 Ball-milled amine and isothiocyanate reactions
2.4 Ball-milled amine thiol reactions
2.5 Ball-milled nitroxide radical coupling reactions
3 Factors impacting ball-milled click chemistry
3.1 Catalysts
3.2 Milling balls
3.3 Additive
3.4 Stoichiometry
3.5 Reaction time
4 Conclusion and outlook
ball mill / click chemistry / mechanochemistry / CuAAc / Diels-Alder / amine and isothiocyanate reaction / amine thiol reaction / nitroxide radical coupling reaction
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
(谷威, 李志强, 朱申敏, 张荻. 化学学报, 2008, (09): 1097.
|
| [5] |
(张修超, 蔡晓兰, 周蕾, 乔颖博, 吴灿, 张爽, 朱伟. 材料导报, 2018, 32(15): 2653.)
|
| [6] |
(张宝剑, 林少芬, 戴乐阳, 刘志杰. 材料导报, 2014, 28(S1): 403.)
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
(许维维. 昆明理工大学硕士论文, 2017.)
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
/
| 〈 |
|
〉 |