 PDF(2638 KB)
PDF(2638 KB)


Research on the Reaction of N,N-Dimethylformamide (DMF) as Synthons
Mina Zhao, Jiayi Tang, Yaodu Zhang
Prog Chem ›› 2025, Vol. 37 ›› Issue (11) : 1661-1673.
 PDF(2638 KB)
PDF(2638 KB)
 PDF(2638 KB)
PDF(2638 KB)
Research on the Reaction of N,N-Dimethylformamide (DMF) as Synthons
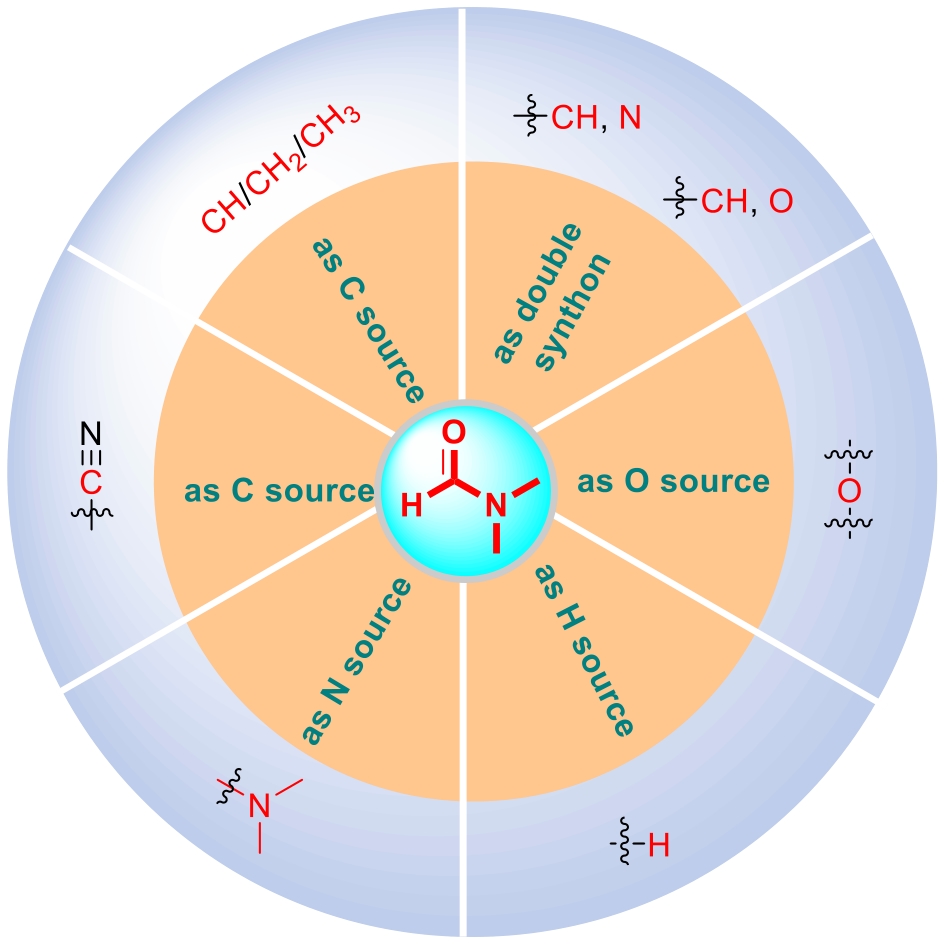
N,N-dimethylformamide (DMF) is a common organic compound. It is not only often used as a solvent in organic reactions but also widely employed as a reaction reagent in industrial production, playing an important role in organic synthesis for a long time. It is worth noting that DMF itself can act as a synthon to provide different structural units for participation in organic synthesis reactions, and it plays a very important role in the construction of complex, diverse and structurally novel functional molecules. Therefore, this review focuses on introducing the performance of DMF as a multifunctional precursor in various reactions, summarizes the latest progress of DMF as an amine source, carbon source, hydrogen source, oxygen source and double synthon reactions, and prospects the future development direction of this field, hoping to provide a reference for the later research on reactions involving DMF as a synthon.
Contents
1 Introduction
2 Reaction of DMF as a synthon
2.1 Reaction of DMF as an amine source
2.2 Reaction of DMF as a carbon source
2.3 Reaction with DMF as a hydrogen source
2.4 Reaction of DMF as an oxygen source
2.5 DMF as a double synthon
3 Conclusion and outlook
N,N-dimethylformamide / synthon / transformation / mechanism / dual function
| [1] |
(徐利军, 李宗军, 韩福社, 高翔, 有机化学, 2024, 44(1): 242).
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
(刁学文, 杨迪, 杨启亮, 蔡小华, 有机化学, 2021, 41(4): 1434).
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
(肖朵朵, 刘海灵, 周鹏, 张建涛, 刘卫兵, 有机化学, 2022, 42(5): 1438).
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(赵咪娜, 杨梓墨, 杨得锁, 有机化学, 2022, 42(1): 111).
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
/
| 〈 |
|
〉 |