 PDF(16902 KB)
PDF(16902 KB)


Modification of Cathode Materials for Prussian Blue-Based Sodium-Ion Batteries
Qingping Li, Tao Li, Chenchen Shao, Wei Liu
Prog Chem ›› 2023, Vol. 35 ›› Issue (7) : 1053-1064.
 PDF(16902 KB)
PDF(16902 KB)
 PDF(16902 KB)
PDF(16902 KB)
Modification of Cathode Materials for Prussian Blue-Based Sodium-Ion Batteries
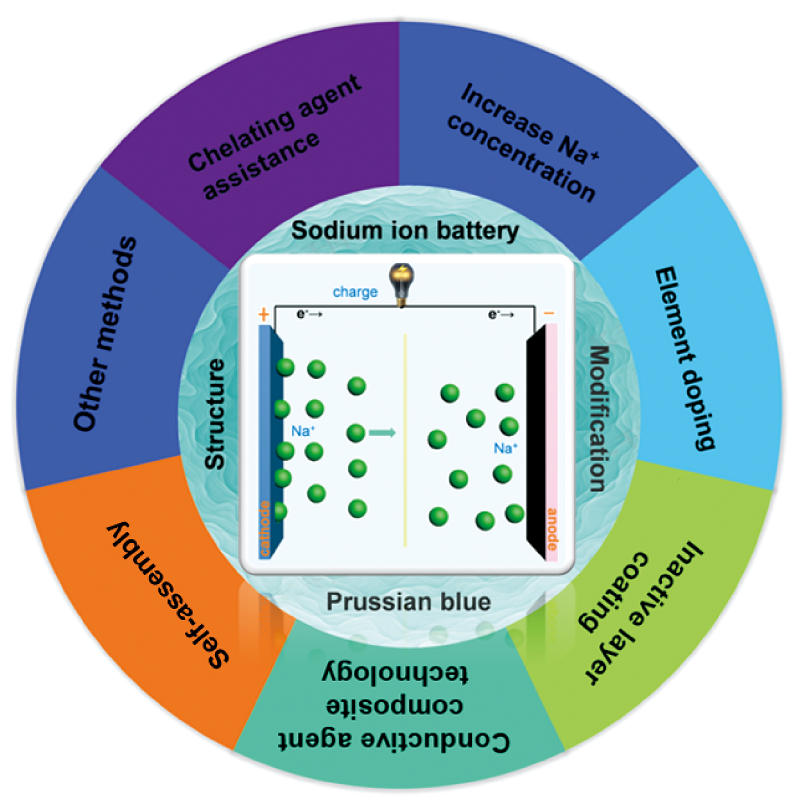
Prussian blue (PB) and its analogues (PBAs), which are composed of three-dimensional frame structure, are ideal cathode materials for sodium ion battery (SIB) and can provide a wide channel for sodium ion embedding and removal. However, there are a lot of water molecules and vacancies in PBAs materials, which greatly reduces the storage sites of sodium ions. Furthermore, transition metal ions in the metal organic framework are easy to dissolve during the cycles, resulting in limited sodium storage capacity and poor cycle stability of PBAs cathode materials. In recent years, a variety of PBAs modification technologies have been developed to improve their sodium storage performance. Based on recent related work and existing literature reports, this paper summarizes the process design, preparation methods, electrochemical behavior and other aspects of different modification technologies, and systematically reviews and prospects the research progress of various modification technologies of PBAs cathode materials in sodium ion batteries.
1 Introduction
2 Structure of Prussian blue and its analogues
3 Modification method of Prussian blue cathode material
3.1 Chelating agent assisted method
3.2 Increase Na+concentration
3.3 Element doping
3.4 Inactive layer coating
3.5 Conductive agent composite technology
3.6 Self-assembly
3.7 Other modification methods
4 Conclusion and outlook
sodium ion battery / prussian blue / cathode material / crystal structure / reaction mechanism / modification method
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
/
| 〈 |
|
〉 |