 PDF(35978 KB)
PDF(35978 KB)


Methanol to Olefins (MTO): A Condensed Matter Chemistry
Nan Wang, Yingxu Wei, Zhongmin Liu
Prog Chem ›› 2023, Vol. 35 ›› Issue (6) : 839-860.
 PDF(35978 KB)
PDF(35978 KB)
 PDF(35978 KB)
PDF(35978 KB)
Methanol to Olefins (MTO): A Condensed Matter Chemistry
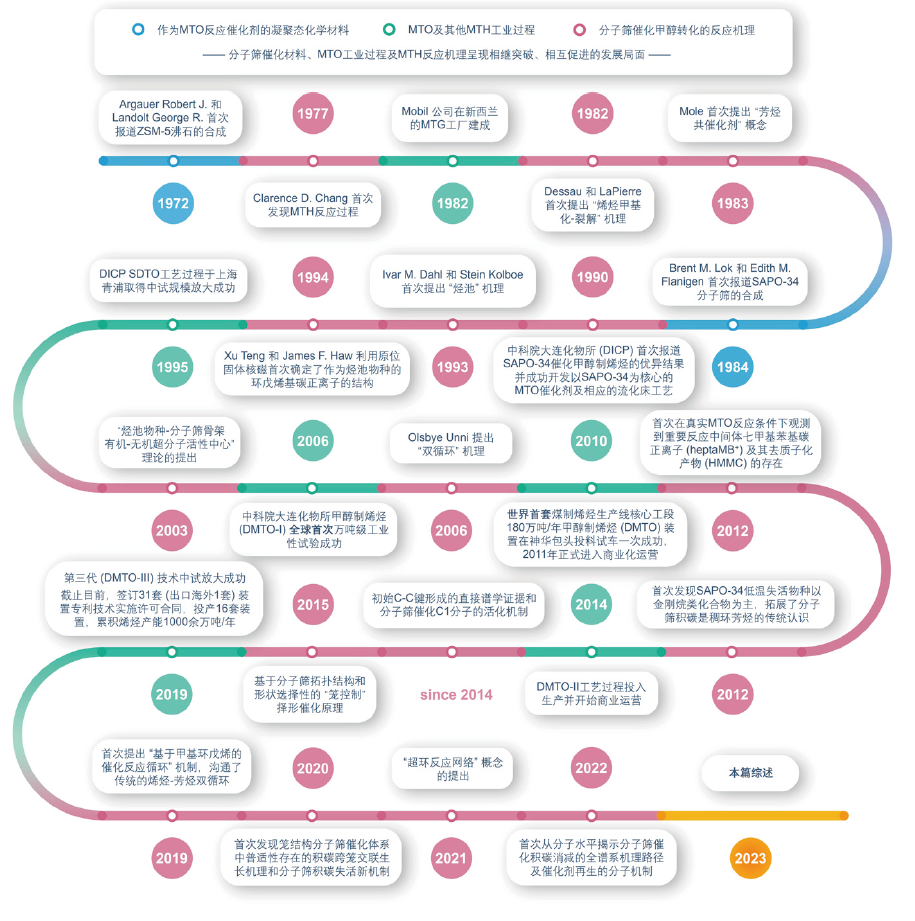
Catalysis is an essential component of condensed matter chemistry, with broad applications in contemporary industrial manufacturing and daily life. Methanol-to-olefins (MTO) reaction, facilitated by condensed-matter porous materials, represents a significant catalytic pathway for the production of light olefins from non-petroleum sources, exemplifying heterogeneous catalytic applications. Investigating reaction mechanisms and catalyst coking/decoking mechanisms is a central focus in catalysis research. The MTO reaction, transpiring within the confined spaces of zeolites and/or molecular sieves, encompasses a dynamic chemical process comprising an induction period, a highly efficient stage, catalyst deactivation, and catalyst regeneration. The formation, evolution, and degradation of active organic species and coke species within the nano-confined spaces of zeolites guide the course of the catalytic reaction. This feature review primarily highlights zeolite/molecular sieve catalysts for the MTO reaction, elucidating the structural-reaction-deactivation relationship based on host-guest chemistry, activation mechanisms of C1 reactants, the catalytic reaction network governed by dynamic mechanisms, chemistries involved in zeolite coking and decoking behavior, as well as the mechanisms of catalyst deactivation and regeneration. The ultimate aim is to provide a profound understanding of condensed matter chemistry in the context of heterogeneous methanol-to-olefins chemistry, thus advancing zeolite catalysis theory and fostering the development of efficient MTO catalysts and high-efficiency, low-carbon catalytic processes under the guidance of condensed matter chemistry.
1 Introduction
2 Catalysts for methanol-to-olefins
2.1 ZSM-5 catalyst with MFI topology structure
2.2 SAPO-34 with CHA topology structure
2.3 Other catalysts with 8-MR pore opening and cavity structure
3 Catalytic reaction mechanism for methanol conversion
3.1 Direct mechanism
3.2 Indirect mechanism
4 Mechanisms of catalyst deactivation/regeneration by zeolite coking/decoking for methanol conversion
4.1 Deactivation mechanism and chemistry involved in zeolite coking
4.2 Regeneration mechanism and chemistry involved in zeolite decoking
5 Conclusions and outlook
condensed matter chemistry / methanol-to-olefins / molecular sieve / reaction mechanism / catalyst deactivation / catalyst regeneration
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
/
| 〈 |
|
〉 |