 PDF(1581 KB)
PDF(1581 KB)


Occurrence of N-Nitrosamines as Harmful Impurities in Pharmaceuticals
Mengru Cao, Zhiwei Ye, Jun Wang, Xiaojin Zhang, Pengfei Lin, Chao Chen
Prog Chem ›› 2023, Vol. 35 ›› Issue (12) : 1881-1894.
 PDF(1581 KB)
PDF(1581 KB)
 PDF(1581 KB)
PDF(1581 KB)
Occurrence of N-Nitrosamines as Harmful Impurities in Pharmaceuticals
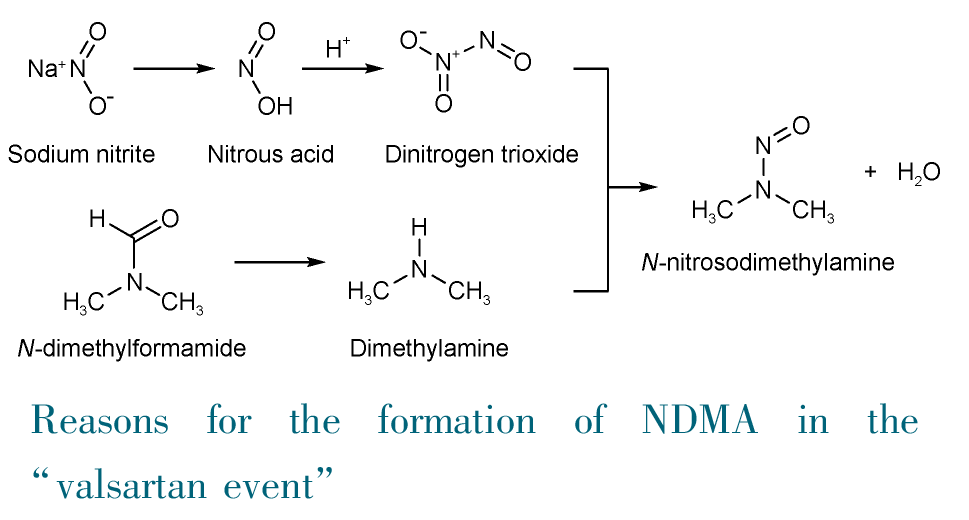
Since the “valsartan event” in 2018, the occurrence of N-nitrosamine as genotoxic impurities in pharmaceuticals has become a hot topic for academia, industry, and the public. N-nitrosamines are a class of strong carcinogens, and more than ten nitrosamines have been included in the International Agency for Research on Cancer (IARC) carcinogen list, with N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) listed as Class 2A carcinogens. In this article, the toxicological characteristics, carcinogenic mechanism, and detection methods of N-nitrosamines are summarized. The causes of nitrosamine impurities in drugs such as valsartan, ranitidine, and metformin are reviewed as well as the regulatory requirements and measures for nitrosamine impurities in drugs in the European Union, the United States, and China. The carcinogenic risk caused by nitrosamine impurities in the above mentioned drugs is estimated. Valsartan has the highest concentration of nitrosamine impurities (NDMA content: not detected~20.19 μg/tablet; NDEA content: not detected~1.31 μg/tablet), resulting in the highest extra cancer risk (CR): the median CR value is 4.69 × 10-6 while the 75th percentile CR value is as high as 5.61 × 10-4 which means at least 25% of tablets can bring high cancer risk. Ranitidine and metformin have much lower nitrosamine impurities, and their cancer risk is close to or below the 10-6 safety level. The carcinogenic risk caused by unqualified pharmaceuticals with nitrosamines impurities is much higher than that caused by nitrosamines in food and drinking water, and even slightly higher than that caused by tobacco nitrosamines. Therefore, the health effect due to unqualified pharmaceuticals needs to be regarded seriously. Since 2020, there is no problem of excessive nitrosamine impurities in raw materials and finished drugs thanks to enhanced administration. This article provides references for professionals from the relevant institutions in the fields of pharmaceutical production, health evaluation, research, and regulation.
1 Introduction
2 Detection of nitrosamine impurities in pharmaceuticals
3 Toxicological properties of nitrosamines
3.1 Basic characteristics
3.2 Carcinogenic mechanism
3.3 Carcinogenic effect factor
3.4 Carcinogenic risk assessment of pharmaceutical nitrosamines
4 Epidemiological investigation
5 Sources of nitrosamine impurities in pharmaceuticals
6 Endogenous production of nitrosamines after administration
7 Comparison with other exogenous exposure pathways
8 Pharmaceutical regulatory measur
N-nitrosamine / pharmaceutical / carcinogenicity / impurity / risk analysis
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
(许后效. 环境科学丛刊, 1980, 02: 16.).
|
| [6] |
EMA. EMA reviewing medicines containing valsartan from Zhejiang Hua hai following detection of an impurity: some valsartan medicines being recalled across the EU. [2018-07-05]. ttps://www.ema.europa.eu/en/news/ema-reviewing-medicines-containing-valsartan-zhejiang-huahai-following-detection-impurity-some.
|
| [7] |
(葛雨琦, 叶晓霞, 乐健, 杨永健, 王彦. 药物分析杂志, 2020, 40(01): 83.).
|
| [8] |
国家药品监督管理局. 国家药监局新闻发言人介绍华海药业缬沙坦原料药有关情况. [2018-07-29]. https://www.nmpa.gov.cn/directory/web/nmpa//yaowen/ypjgyw/20180729174001758.html.
|
| [9] |
|
| [10] |
FDA. FDA publishes a list of valsartan-containing products not part of the recall Update. [2018-07-24]. https://www.fda.gov/.
|
| [11] |
EMA. EU authorities take further action in ongoing review of sartans: Zheijiang Huahai placed under increased supervision; Aurobindo Pharma stopped from supplying irbesartan to the EU. [2018-10-15]. https://www.ema.europa.eu/en/news/eu-authorities-take-further-action-ongoing-review-sartans-zheijiang-huahai-placed-under-increased.
|
| [12] |
FDA. Statement alerting patients and health care professionals of NDMA found in samples of ranitidine. [2019-09-13]. https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine.
|
| [13] |
|
| [14] |
|
| [15] |
FDA. Statement from Janet Woodcock, M.D., director of FDA’s Center forDrug Evaluation and Research, on impurities found in diabetes drugs outside the U.S.A. [2019-12-05]. https://www.fda.gov/news-events/press-announcements/statement-janet-woodcock-md-director-fdas-center-drug-evaluation-and-research-impurities-found.
|
| [16] |
FDA. FDA Requests Removal of All Ranitidine Products (Zantac) from the Market. [2020-04-01]. https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market.
|
| [17] |
FDA. Control of Nitrosamine Impurities in HumanDrugs. [2021-02-24]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs.
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
国家药典委员会. 关于缬沙坦国家标准修订稿的公示(第二次). [2019-01-02]. https://ibook.antpedia.com/x/243596.html.
|
| [23] |
MCLS. GC -MS Method for the determination of NDMA and NDEA in Sartan (Valsartan, Losartan, Candesartan, Irbesartan, Olmesartan) APIs (developed on a Shimadzu GC-MS QP 2020plus). https://www.the-mcls.org/.
|
| [24] |
FDA. Combined Headspace N-Nitrosodimethylamine (NDMA), NNitrosodiethylamine (NDEA), N-Nitrosoethylisopropylamine (NEIPA), and N-Nitrosodiisopropylamine(NDIPA) Impurity Assay by GC-MS/MS. [2019-04-29]. https://www.fda.gov/media/124025/download.
|
| [25] |
FDA. Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS) Method for theDetermination of Six Nitrosamine Impurities in ARBDrugs. [2019-05-21]. https://www.fda.gov/media/125478/download.
|
| [26] |
FDA. Laboratory Analysis of Valsartan Products. Laboratory Analysis of Valsartan Products. [2021-07-01]. https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-valsartan-products.
|
| [27] |
(侯金凤, 李珉, 寇晋萍, 刘海涛, 车宝泉, 李文东. 中国药学杂志, 2021, 56(10): 842.).
|
| [28] |
|
| [29] |
(范婷婷, 张晓栋. 医学食疗与健康, 2019, 15: 16.).
|
| [30] |
ChenD,
(陈丹, 汪嘉丽. 中国药物经济学, 2022, 17(07): 97.).
|
| [31] |
(葛雨琦, 叶晓霞, 乐健, 杨永健, 王彦. 中国医药工业杂志, 2020, 51(06): 759.).
|
| [32] |
(彭燕, 杨云帆, 赵兰玲, 杜金蔚, 林妮妮, 董颖. 中国药物评价, 2022, 39(03): 199.).
|
| [33] |
(刘晶晶, 梁智渊, 梁爱仙, 钟丹敏, 刘敏, 王铁杰, 李玉兰. 中国现代应用药学, 2023, 40(09): 1224.).
|
| [34] |
(李尚颖, 刘慧颖, 张丽, 徐万魁. 品牌与标准化, 2023, 379(02): 113.).
|
| [35] |
FDA. Laboratory Analysis of Metformin Products. [2021-07-01]. https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-metformin.
|
| [36] |
(陈鸿玉, 李昭, 刘波, 刘雁鸣, 李昌亮, 兰文, 李帅, 王晓菲. 华西药学杂志, 2022, 37(05): 536.).
|
| [37] |
FDA. Laboratory Analysis of Metformin Products. [2021-07-01]. https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-metformin.
|
| [38] |
(刘博, 张佟, 黄露, 张庆生, 范慧红. 中国药物警戒, 2021, 18(05): 454.).
|
| [39] |
(徐艳梅, 李挥, 张素平, 乔晓宁, 苗会娟, 盖成, 王茉莉, 高燕霞. 化学分析计量, 2022, 31(05): 28.).
|
| [40] |
(郭常川, 刘琦, 张雷, 郑静, 汪勇, 杨书娟, 褚志杰, 牛冲, 徐玉文. 色谱, 2020, 38(11): 1288.).
|
| [41] |
(周刚, 李鹏飞, 高家敏. 中国抗生素杂志, 2021, 46(04):291.).
|
| [42] |
|
| [43] |
|
| [44] |
(杨竹, 杭太俊, 郭晓迪, 田芸, 曹伟. 药学与临床研究, 2020, 28(04): 270.).
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
(边丽. 兵团教育学院学报, 2001, 01: 55.).
|
| [56] |
(陆婷婷, 朱小芳, 林东翔, 李燕, 周蓉. 广东化工, 2021, 48(23): 88.).
|
| [57] |
(顾维雄. 化学教育, 1992, 05: 9.).
|
| [58] |
(朱雨霏. 环境保护与循环经济, 2008, 05: 34.).
|
| [59] |
OEHHA. Final Statement of Reasons 22 California Code of Regulations. 1989. [2021-07-01]. https://oehha.ca.gov/.
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
国家药典委员会. 关于《中国药典》2020年版四部通则增修订内容(第四批)的公示-国家药典委员会. [2019-01-23]. https://www.chp.org.cn/gjyjw/tongze/3282.jhtml.
|
| [64] |
(闾春林, 刘永东, 王云海, 钟儒刚. 化学学报, 2007, 65(16): 1568.).
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
FDA. Statement alerting patients and health care professionals of NDMA found in samples of ranitidine. [2018-12-11]. https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine.
|
| [73] |
(李晓, 贝尔, 汪隽, 徐志祥, 陈超. 中国给水排水, 2018, 34(22): 13.
|
| [74] |
|
| [75] |
|
| [76] |
(关玥. 上海交通大学硕士论文, 2018.).
|
| [77] |
(罗曼. 中国疾病预防控制中心硕士论文, 2020.).
|
| [78] |
(罗琼. 华中科技大学硕士论文, 2021.).
|
| [79] |
|
| [80] |
Committee for Medicinal Products for Human Use. Guidelines on the limits of genotoxic impurities. London: European Medicines Agency, 2006.
|
| [81] |
M7 Assessment and Control ofDNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk; International Conference on Harmonisation; Guidance for Industry; Availability. The Federal Register. Washington: Federal Information & NewsDispatch, LLC, 2015. 30465.
|
| [82] |
国家药品监督管理局药品审评中心. 国家药监局药审中心关于发布《化学药物中亚硝胺类杂质研究技术指导原则(试行)》的通告(2020年第1号). [2020-05-08]. https://www.nmpa.gov.cn/yaopin/ypggtg/ypqtgg/20200508160101122.html.
|
/
| 〈 |
|
〉 |