 PDF(14526 KB)
PDF(14526 KB)


Application of MOFs-Derived Metal Oxides in Catalytic Total Oxidation of VOCs
Tao Peng, Qianqian Chai, Chuanqiang Li, Xuxu Zheng, Lingjuan Li
Prog Chem ›› 2024, Vol. 36 ›› Issue (1) : 81-94.
 PDF(14526 KB)
PDF(14526 KB)
 PDF(14526 KB)
PDF(14526 KB)
Application of MOFs-Derived Metal Oxides in Catalytic Total Oxidation of VOCs
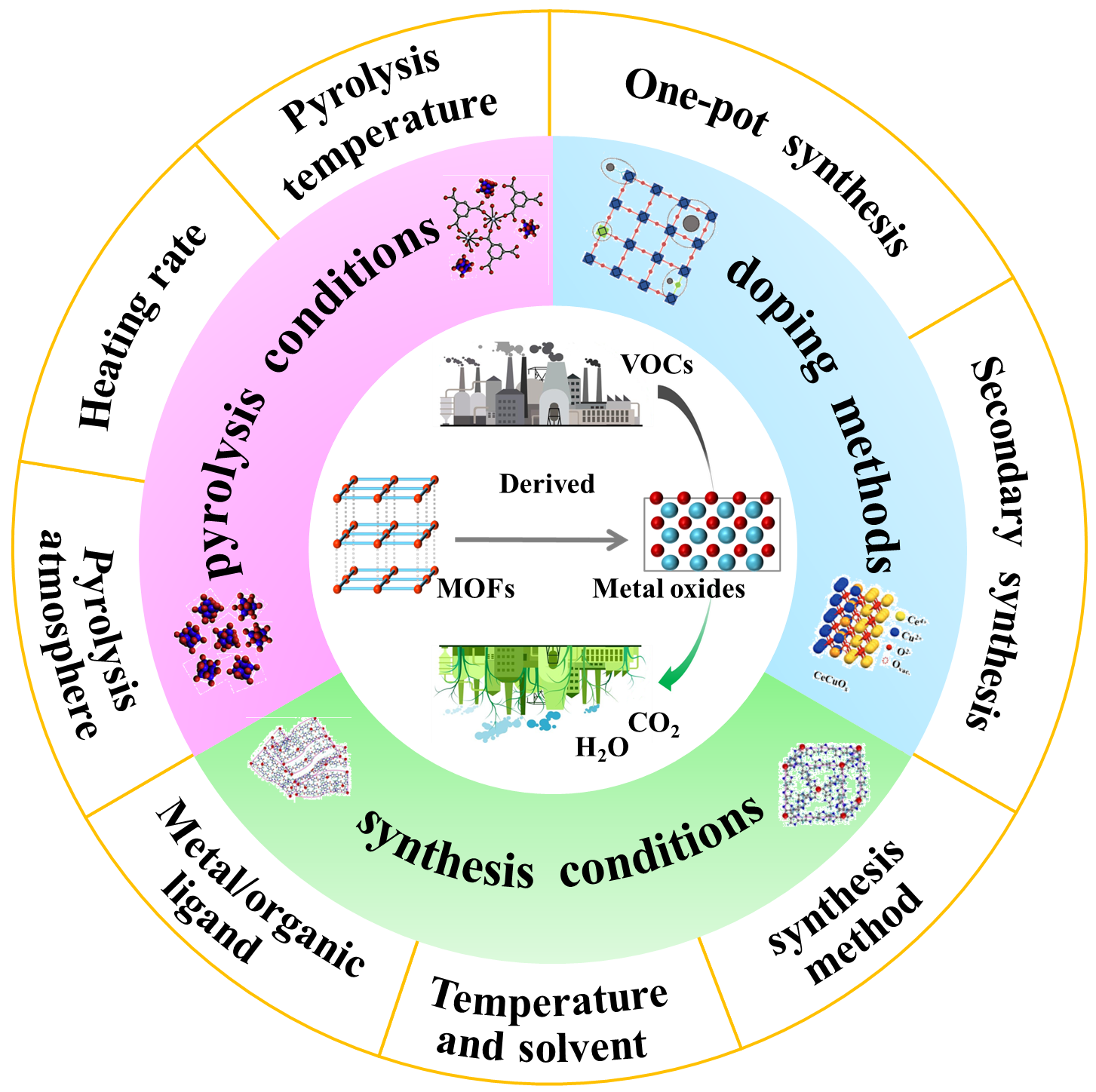
The emission of a significant amount of VOCs has resulted in severe impacts on both human health and the environment. Currently, the most effective method for treating VOCs is their total oxidation to carbon dioxide and water through metal oxide catalysis. To enhance the catalytic performance of metal oxides, various synthetic strategies have been developed, including morphology, defect, and doping engineering. However, these processes are cumbersome and require further improvements to enhance the catalytic performance. On the other hand, metal-organic frameworks (MOFs)-derived metal oxides have been extensively used to catalyze the complete oxidation of VOCs. This is because of their tunable morphology, large specific surface area, high defect concentration, and excellent doping dispersion. However, there is a lack of a comprehensive summary of the application of MOFs-derived metal oxides in the total oxidation of VOCs. Therefore, this paper reviews the synthesis conditions, doping methods, and pyrolysis conditions of MOFs from the control strategy of derived metal oxides. It also summarizes the regulation methods and the relationship between the physicochemical properties of derived metal oxides and the total oxidation performance of VOCs. Additionally, this paper discusses the future development and challenges of MOFs-derived metal oxides.
1 Introduction
2 Regulatory strategies of MOFs-derived metal oxides and their application in catalytic total oxidation of VOCs
2.1 Synthesis conditions
2.2 Doping methods
2.3 Pyrolysis conditions
3 Mechanism of catalytic VOCs total oxidation
4 Conclusion and outlook
Metal-organic frameworks(MOFs) / Metal oxide / Nanomaterials / Volatile organic compounds (VOCs) / Total oxidation
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
/
| 〈 |
|
〉 |