 PDF(2996 KB)
PDF(2996 KB)


Saccharide Sensors Based on Phenylboronic Acid Derivatives
Tan Shi, Donghui Kou, Yanan Xue, Shufen Zhang, Wei Ma
Prog Chem ›› 2024, Vol. 36 ›› Issue (1) : 106-119.
 PDF(2996 KB)
PDF(2996 KB)
 PDF(2996 KB)
PDF(2996 KB)
Saccharide Sensors Based on Phenylboronic Acid Derivatives
Phenylboronic acid, a kind of synthetic molecule that can covalently bind with saccharide, has attracted wide attention in the field of saccharide detection. It has the characteristics of good stability, strong recognition ability and easy coupling with various detection systems. In this paper, the mechanism of phenylboronic acid binding to saccharide and its specific applications in detection was first introduced. What’s more, the strategies for structural modification, in the manner of introducing electron-withdrawing group or electron-donating group into ortho, meta and para position of the boric acid group on the benzene ring, were mainly discussed, and the progress made in reducing pKa and improving the selectivity according to these strategies were summarized. At the same time, the saccharide sensors based on these new phenylboronic acid derivatives in recent years were also summarized, including electrochemical sensors, fluorescence sensors, gels/microgels and photonic crystals, and their detection principles were discussed. The main analytes are monosaccharides with similar structures, such as glucose and fructose. Finally, the research of these sensors based on phenylboronic acid derivatives was compared, and their advantages and disadvantages were analyzed. Meanwhile, the applications of saccharide sensors based on phenylboronic acid derivatives in the future are prospected from two aspects including the integration of diagnosis and treatment and the identification of saccharide in complex chemical environment.
1 Introduction
2 Phenylboronic acid and its derivatives
2.1 Reaction principle of phenylboronic acid and saccharides
2.2 Structural modification strategy of phenylboronic acid
2.3 Detection principle of saccharides in phenylboronic acid
3 Saccharide sensors based on phenylboronic acid derivatives
3.1 Electrochemical sensors for saccharide detections
3.2 Fluorescent sensors for saccharide detections
3.3 Photonic crystals for saccharide detections
3.4 Gels for saccharide detections
4 Conclusion and outlook
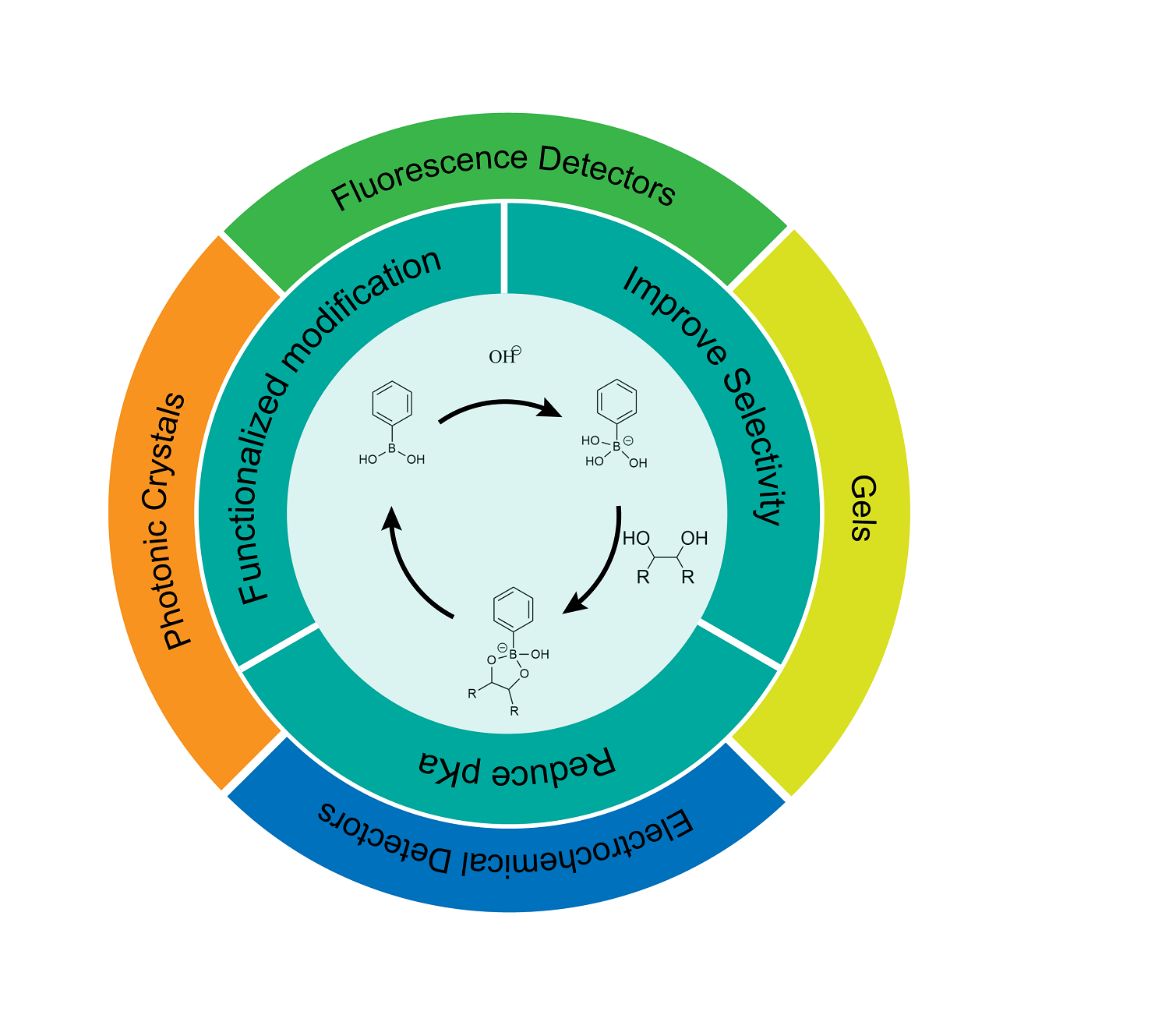
phenylboronic acid / saccharide / electrochemistry / fluorescence / gel / photonic crystal
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
(王卓, 李朔. 大学化学, 2020, 35(7): 95.).
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
/
| 〈 |
|
〉 |