 PDF(2617 KB)
PDF(2617 KB)


Catalytic Conversion of Hydroxyl Compounds : Conversion of Phenols and Alcohols to Ethers and Esters
Xiaoyu Wang, Ruiyi Wang, Xiangpeng Kong, Yulan Niu, Zhanfeng Zheng
Prog Chem ›› 2024, Vol. 36 ›› Issue (3) : 335-356.
 PDF(2617 KB)
PDF(2617 KB)
 PDF(2617 KB)
PDF(2617 KB)
Catalytic Conversion of Hydroxyl Compounds : Conversion of Phenols and Alcohols to Ethers and Esters
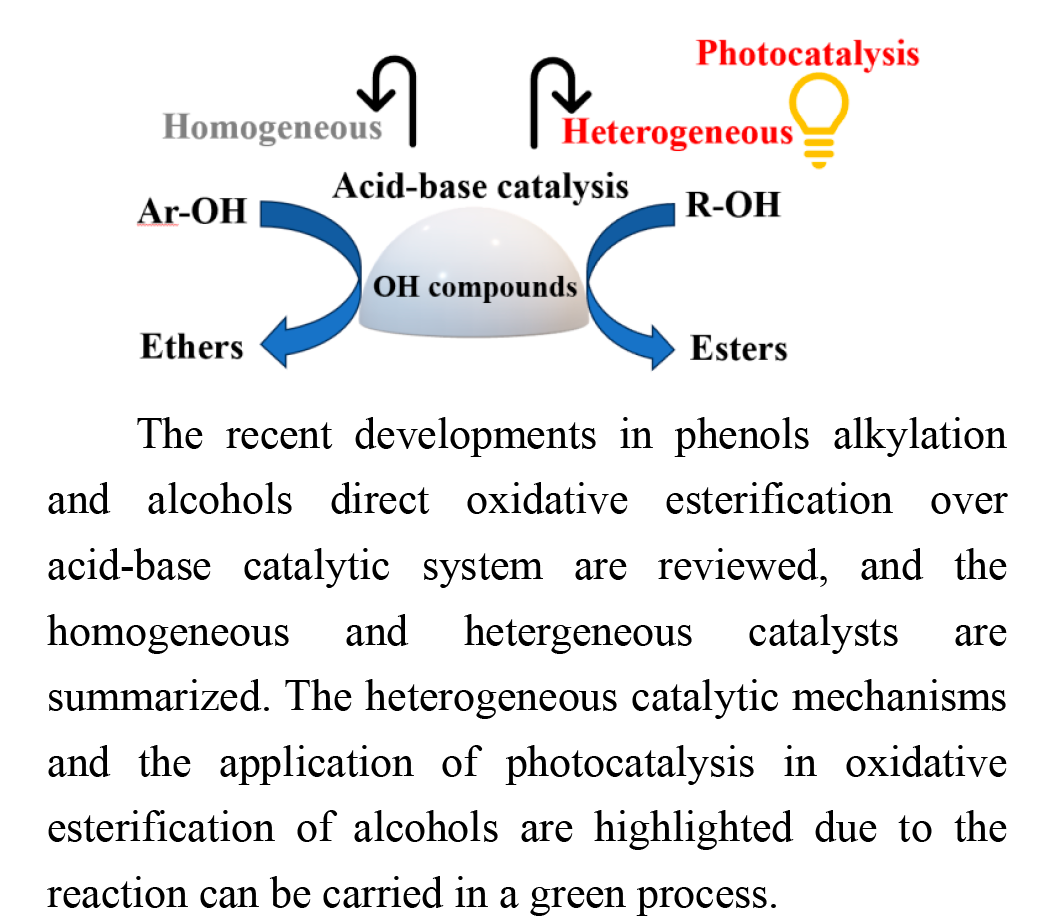
With the background of rapid economic development, the green synthesis of high-value-added chemicals has attracted great interest. Ethers and Esters, the products of hydroxyl compound conversion, are important green chemical products. However, the harsh reaction conditions limit their application. Herein, we review the developments in the catalysis of phenols alkylation to ethers and alcohols oxidative esterification to esters. The modification strategy and catalytic mechanism of the catalytic systems are summarized. The heterogeneous catalytic system and its mechanisms have been mainly discussed. It is found that the acid-base synergistic catalysis and the synergistic effect between metal and support are favorable for the green synthesis of ethers and esters under mild reaction condition. Besides, the application of photocatalysis in oxidative esterification of alcohols is highlighted because the photocatalytic reaction is considered a promising green synthesis method. Finally, the research on the catalytic conversion of hydroxyl compounds are summarized and prospected, and we believe that the synthesis and modification of new catalysts and the exploration of catalytic mechanisms is still a promising research field.
1 Introduction
2 Activation of phenols hydroxyl group:alkylation of phenols
2.1 Homogeneous catalyst
2.2 Heterogeneous catalyst
2.3 Alkylating agent
2.4 Catalytic mechanism of phenols alkylation
3 Activation of alcohols hydroxyl group:oxidative esterification of alcohols
3.1 Homogeneous catalyst
3.2 Heterogeneous catalyst and catalytic mechanism
4 Photocatalytic oxidative esterification of alcohols
5 Conclusions and outlook
hydroxyl compounds / catalytic conversion / heterogeneous catalysis / phenols alkylation / alcohols oxidative esterification / photocatalysis
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
/
| 〈 |
|
〉 |