 PDF(12539 KB)
PDF(12539 KB)


The Space Confinement Effect of Catalytic Materials and Its Application in Low Temperature Denitration
Wei Zhang, Qiao Wu, Yehao Fu, Yaocheng Liang, Min Ruan, Yanshan Yin, Shan Cheng
Prog Chem ›› 2024, Vol. 36 ›› Issue (6) : 928-938.
 PDF(12539 KB)
PDF(12539 KB)
 PDF(12539 KB)
PDF(12539 KB)
The Space Confinement Effect of Catalytic Materials and Its Application in Low Temperature Denitration
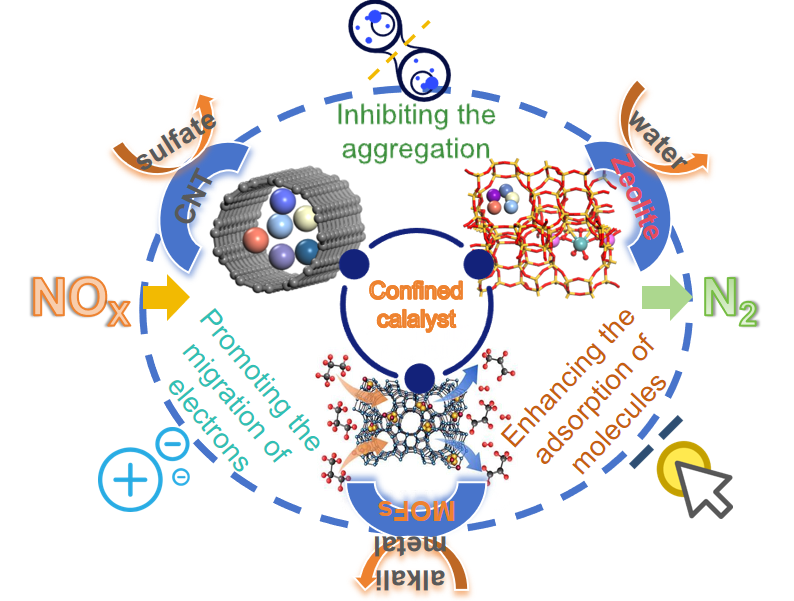
The spatial confinement effect of porous materials can change the surface electron distribution and electron transport performance,realize the local reaction in the micro-nano pore domain,effectively prevent the external environment from affecting the active substances in the confined space,and inhibit the agglomeration of the active center,which is an effective way to strengthen the denitrification performance of the catalyst.This paper focuses on the changes in surface energy,periodic boundary conditions and electronic energy levels of different catalytic materials,and discusses the formation mechanism of the spatial confinement effect.The effects of confinement effect on the dispersion of active species,redox ability and molecular adsorption strength in the reaction process and the regulation strategies of size effect,encapsulation effect and molecular sieve effect in confinement effect were described.The strengthening effects of confined catalysts on NH3adsorption performance,reaction selectivity,anti-toxicity and denitrification activity in the denitrification process were summarized.Finally,the development prospect of confined denitrification catalysts was prospected.
1 introduction
2 The influence of space confinement effect on catalytic reaction
2.1 Inhibiting the aggregation of active species
2.2 Promoting the migration of interface electrons
2.3 Enhancing the adsorption of reaction molecules
3 Spatial confinement effect regulation strategy
3.1 Control of confinement size effect
3.2 Control of confinement encapsulation effect
3.3 Control of confined molecular sieve effect
4 The application of spatial confinement effect in low-temperature denitrification
4.1 Strengthen the adsorption performance of NH3
4.2 Enhance the selectivity of the denitration reaction
4.3 Enhance the anti-toxicity of the catalyst
5 Conclusion and outlook
porous materials / confinement effect / catalytic mechanism / regulation strategy / low-temperature denitration
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
Ekpe, Ikenna. Int. Res. J. Environmental Sci., 2017, 6(10): 45.
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
/
| 〈 |
|
〉 |