 PDF(20545 KB)
PDF(20545 KB)


Synthesis of 4-Monosubstituted 1,2,3-Triazoles
Liguo Teng, Shuitao Zhang, Tiebo Xiao, Baomin Yang, Yubo Jiang
Prog Chem ›› 2024, Vol. 36 ›› Issue (7) : 1014-1025.
 PDF(20545 KB)
PDF(20545 KB)
 PDF(20545 KB)
PDF(20545 KB)
Synthesis of 4-Monosubstituted 1,2,3-Triazoles
1,2,3-Triazoles are a kind of five-membered N-heterocycles featured with unique properties in biology and material science.They have played more and more important roles in the fields of medicine,pesticide,and materials since the Cu-catalyzed diploar cycloaddition reaction of azide and alkyne was founded by Sharpless and Medal groups,respectively.4-Monosubstituted 1,2,3-triazole is considerably important in its family,especially because of its structural merits of widely facile modifications.The synthesis of 4-monosubstituted 1,2,3-triazoles based on different reaction types and substrates,including cycloaddition of alkyne with azide,cycloaddition of nitroolefin with azide,cyclization of hydrazone with amino derivatives,and N1-substituent removal of 1,4-disubstituted 1,2,3-triazoles are reviewed.The substrate scope,limitation,and representative mechanism are also discussed 。
1 Introduction
2 Progress in the synthesis of 4-monosubstituted 1,2,3-triazoles
2.1 1,3-Dipolar cycloaddition of alkyne and azide
2.2[3+2]Cycloaddition of nitroolefin and azide
2.3 Cyclization of hydrazone with amino derivative
2.4 Removal of N1-substituent from 1,4-disubstituted 1,2,3-triazole
2.5 Reaction of alkenyl bromide with sodium azide
2.6 Other methods
3 Conclusion and outlook
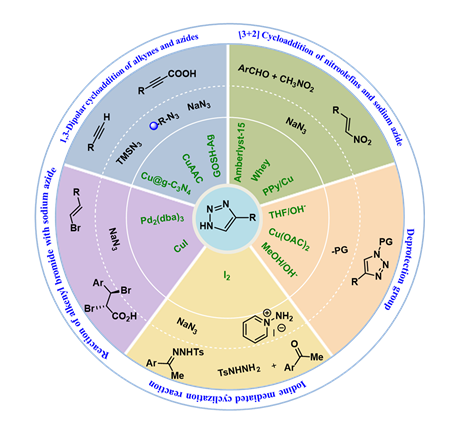
4-monosubstituted 1,2,3-triazoles / synthesis / cycloaddition / cyclization / substituent removal
| [1] |
(王景梅, 李凌君, 张贵生. 有机化学, 2009, 29(1): 13.).
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
(肖琳霞, 石德清. 有机化学, 2010, 30(1): 85.).
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
(刘家健. 中国抗生素杂志, 2006, 31(2): 100.)
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
(张文生, 匡春香, 杨青. 有机化学, 2011, 31(1): 54.).
|
| [27] |
(江玉波, 殷云川, 陈润奇, 雷鹏飞, 肖付俊. 化学研究与应用, 2014, 26(11): 1695.).
|
| [28] |
(江玉波, 赵粉, 韩春美, 梁雪秋, 杨睿. 化学研究与应用, 2014, 26(12): 1831.).
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
(王亮, 潘亮, 陈群, 何明阳. 常州大学学报(自然科学版), 2017, 29(4): 1.).
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
/
| 〈 |
|
〉 |