 PDF(60752 KB)
PDF(60752 KB)


The Electronic Principle of Nanomaterial Surface Chemistry
Guolei Xiang
Prog Chem ›› 2024, Vol. 36 ›› Issue (6) : 851-866.
 PDF(60752 KB)
PDF(60752 KB)
 PDF(60752 KB)
PDF(60752 KB)
The Electronic Principle of Nanomaterial Surface Chemistry
Revealing The intrinsic electronic principles driving the surface chemistry of nanomaterials is a central goal in nanoscience;however,the concepts and theoretical frameworks have long remained incomplete and unsystematic.this review systematically introduces a theoretical framework to reveal the interaction mechanisms and trends of surface ligands with nanomaterials at the electronic level,on the basis of competitive orbital redistribution in chemisorption and a concept of orbital potential,the characteristic electronic attribute directly determining surface reactivity.Based on the competitive interactions between surface coordination bonds and bulk energy bands,This theoretical framework can provide coherent answers to these key scientific issues.(1)the opposite and uniform relation of surface activity and stability in nanomaterials originates from the normalization principle of wavefunctions.(2)the physical nature of enhanced surface activity by size reduction lies in two mechanisms:weakening the constrain strength to surface valence atomic orbitals by nanomaterial energy bands,and amplifying the effects of other structural parameters like defects.(3)Nanoscale cooperative chemisorption(NCC)model generally reveals the electronic-level mechanisms and common rules how ligand coverage regulates the energy band states and physical/chemical properties of nanomaterials.(4)the roles and interaction mechanisms of nanomaterial size(r),specific surface area(S/V),surface ligands,and ligand coverage(θ)in nanomaterial surface chemical reactions are elucidated.
1 Introduction
2 Nanomaterial surface chemistry
2.1 Key science issues
2.2 Three types of understanding viewpoints
2.3 Nanomaterial surface coordination chemistry
2.4 Four modes of nanomaterial surface effects
3 Electronic principle of structure-function relationships
3.1 Structure-function relationship in physical science
3.2 Electronic attributes
3.3 Quantum size effect
4 Chemisorption model based on competitive orbital redistribution
4.1 Chemisorption interaction
4.2 Competitive redistribution of surface valence orbitals
4.3 Orbital potential
4.4 Structure-function relationship of surface reactivity
5 Electronic principle of size-dependent surface reactivity
5.1 Meaning of surface activity
5.2 Mathematic model of surface reactivity
5.3 Dual roles of size reduction in enhancing surface reactivity
6 Nanoscale competitive chemisorption model
6.1 Relationship of energy band and surface reactivity
6.2 Nanoscale competitive chemisorption model
6.3 The roles of r,S/V,andθin nanosurface chemistry
6.4 Two-electronic-state competition model
6.5 The uniform principle of ligand effect on photoluminescence
7 Comparison of typical adsorption models
7.1 Adsorption isotherm model
7.2 Electronic model of chemisorption
7.3 Chemisorption model of nanomaterial
8 Summary and outlook
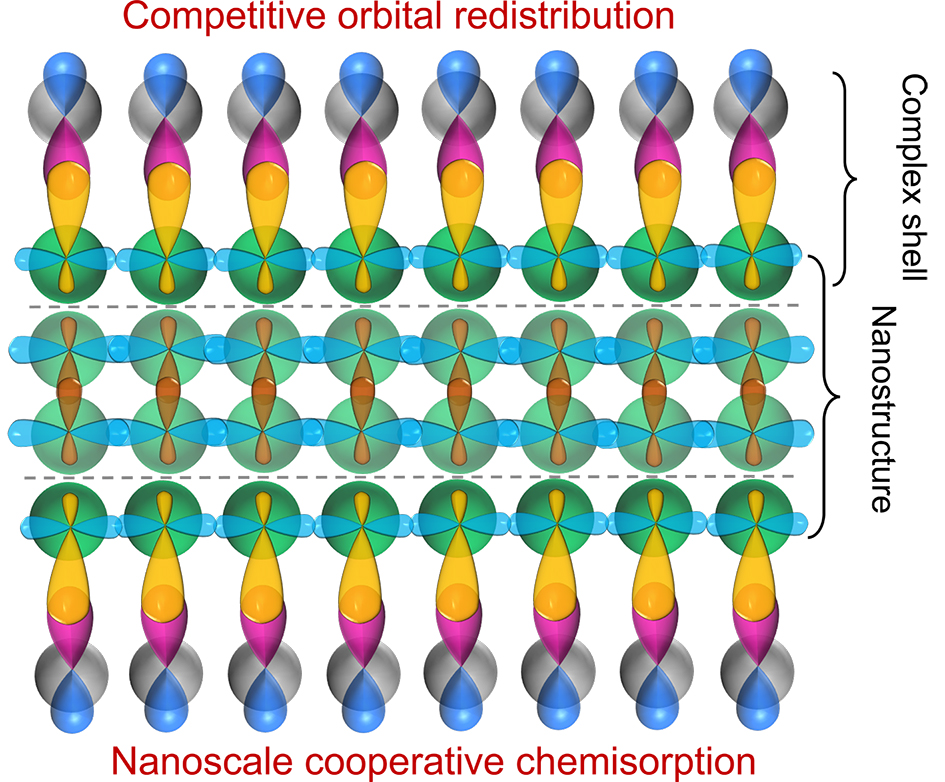
nanomaterial surface chemistry / orbital redistribution / size effect / surface effect / electronic principle / nanoscale cooperative chemisorption
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
(秦瑞轩, 邓果诚, 郑南峰. 化学进展, 2020, 32: 1140.)
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
/
| 〈 |
|
〉 |