 PDF(18337 KB)
PDF(18337 KB)


High-Voltage Tolerant Electrolyte for Lithium-Ion Batteries
Luoqian Li, Mumin Rao, Hong Chen, Shijun Liao
Prog Chem ›› 2024, Vol. 36 ›› Issue (10) : 1456-1472.
 PDF(18337 KB)
PDF(18337 KB)
 PDF(18337 KB)
PDF(18337 KB)
High-Voltage Tolerant Electrolyte for Lithium-Ion Batteries
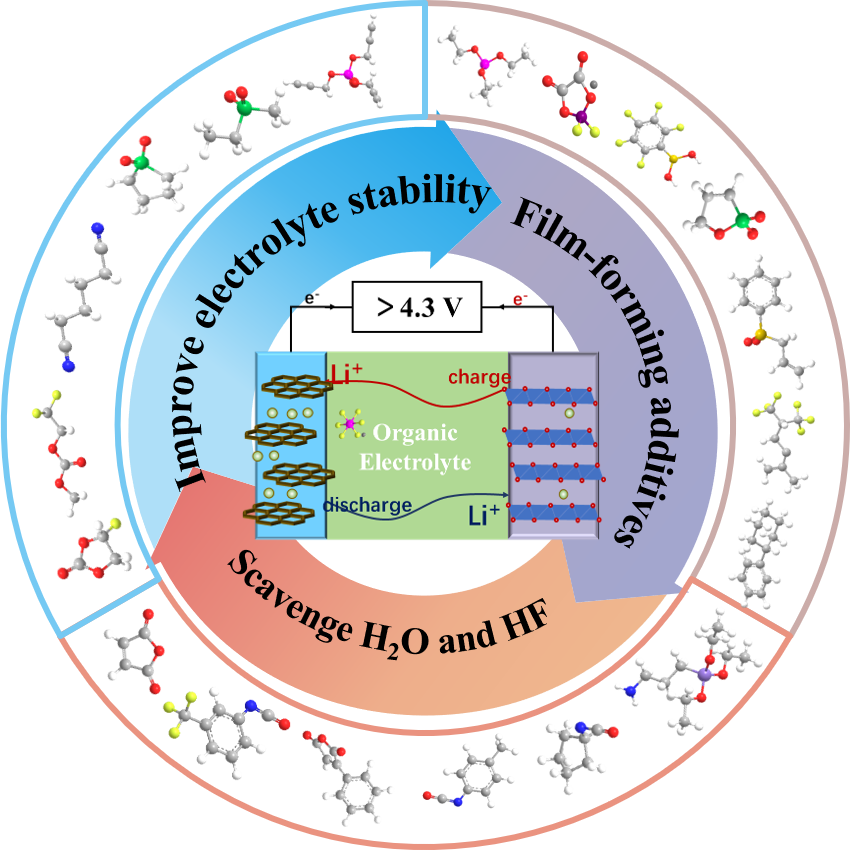
With the rapid development of consumer intelligent electronic devices and electric vehicles, the development of lithium-ion batteries with high energy density has become a very urgent and important issue. Using high-voltage electrode materials and enhancing the work voltage of batteries is an effective pathway to realize the high energy density of battery. However, the conventional carbonate-based electrolyte will undergo oxidation reactions when the voltage is higher than 4.3 V, which will lead to electrolyte decomposition, and finally resulting in the failure of the battery. Actually, it has become one of the main bottlenecks in the development of high-voltage batteries. In order to solve this problem, researchers have carried out a lot of exploration in the design of high-voltage electrolyte in recent years, and made many important research achievements. This review introduces the failure mechanism of batteries under high voltage, and focuses on the strategies and research progress in suppressing high voltage failure from the perspective of electrolytes in recent years, indicates the challenges still existing in the design of high-voltage electrolyte, and finally prospects the future developments of high voltage lithium-ion battery electrolyte.
Contents
1 Introduction
2 Failure mechanism of high-voltage batteries
2.1 Electrolyte decomposition
2.2 Transition metal ion leaching
2.3 HF erosion
3 Progress on high-voltage electrolyte
3.1 Improvement of intrinsic stability of electrolyte
3.2 Construction of stable CEI Layer
3.3 Scavenge H2O and HF
4 Conclusion and outlook
lithium-ion batteries / high voltage electrolyte / solvent / additive / failure mechanism
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
/
| 〈 |
|
〉 |