 PDF(37082 KB)
PDF(37082 KB)


Research Progress of Pseudo-Proteins as Drug Carriers
Xuan Zhang, Min Sun, Yunjiao Xue, Yuhuan Chen, Jing Fang, Fang Yang
Prog Chem ›› 2025, Vol. 37 ›› Issue (2) : 211-225.
 PDF(37082 KB)
PDF(37082 KB)
 PDF(37082 KB)
PDF(37082 KB)
Research Progress of Pseudo-Proteins as Drug Carriers
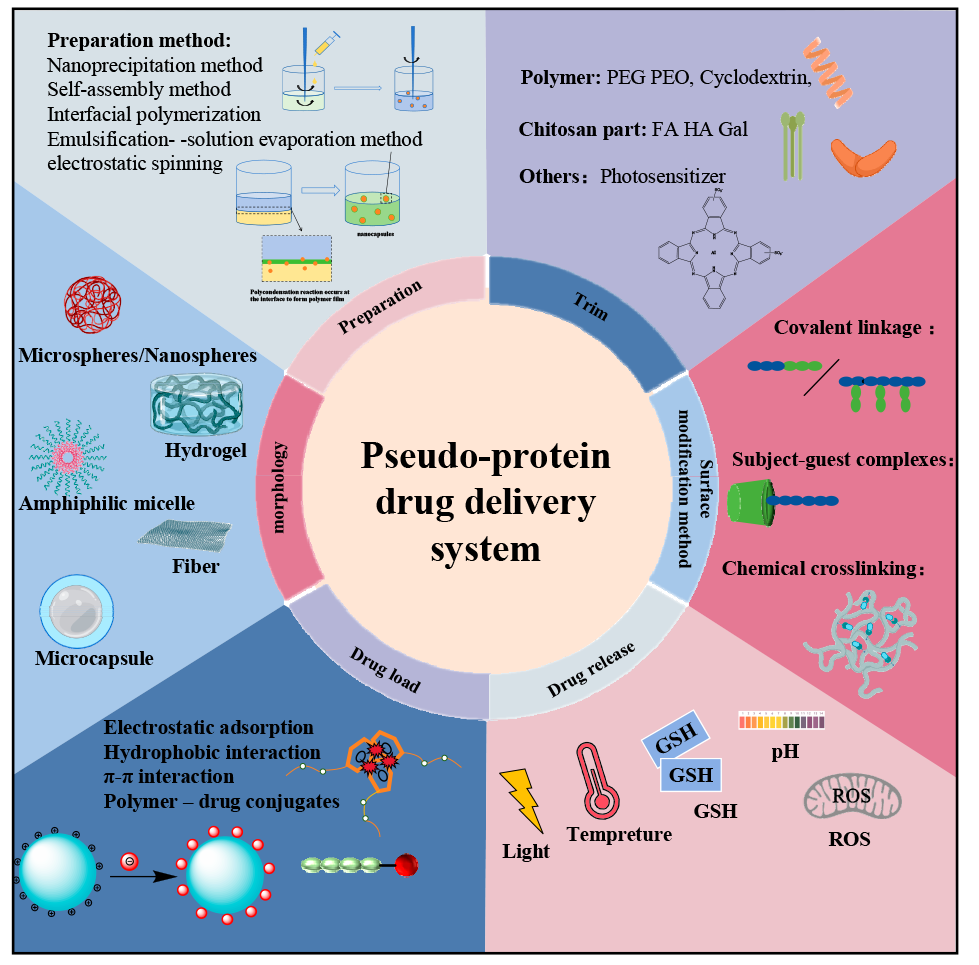
Pseudo-protein materials have the advantages of high biocompatibility, biodegradability, and high tunability, and have attracted wide attention in the biomedical field as a drug carrier in recent years. Pseudo-protein molecules contain amide bonds, ester bonds and other active groups, compared with protein, not only retain the advantages of high tissue compatibility, and ester bonds and other active groups overcome the disadvantages of single protein structure, single function, make it have better mechanical properties and functionality, according to the actual demand for diversified morphology design and surface modification. The pseudo-protein drug carriers constructed by various methods such as self-assembly not only enhance the bioavailability of the drug in vivo, but also make the pseudo-protein drug carriers show ideal targeted controlled release performance with the help of specific signals at the focus. This paper focuses on the pseudo-protein drug delivery materials, introduces the construction and loading mode of pseudo-protein drug carriers, and summarizes the targeted release strategy of pseudo-protein drug carrier, and finally makes the prospect of pseudo-protein in the direction of controlled drug release, so as to provide reference for the subsequent research of pseudo-protein drug carriers.
1 Introduction
2 Construction of pseudo-protein drug carriers
2.1 The pseudo-protein itself constructs the drug carrier
2.2 Pseudo-protein with other substances to construct the drug carriers
3 Drug loading mode of pseudo-protein drug carrier
3.1 Physical coating
3.2 Preparation of sudden-release microcapsules
3.3 Chemical bonding
4 Targeted release of pseudo-protein drug carriers
4.1 Passive targeting
4.2 Active targeting
4.3 Stimulus-responsive targeting
pseudo-proteins / polymer drug carrier / drug loading / targeted release / stimulus response
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
(丁晶晶, 倪鑫炯, 杨成, 孙亚娟. 日用化学工业, 2021, 51(8): 711.)
|
| [50] |
|
| [51] |
(李艳, 罗成, 周婕. 中国生物医学工程学报, 2017, 36(4): 483. )
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
(原焱焱. 有机化学研究, 2023, 11(1): 17.)
|
| [63] |
|
| [64] |
(唐良健, 黄绍德, 王繁盛, 吕焕能, 吴建婷, 黄雪秋, 韦雪琴. 化学试剂, 2023, 45(04): 63.)
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
/
| 〈 |
|
〉 |