 PDF(9832 KB)
PDF(9832 KB)


Research Process on Photoinduced Copper-Catalyzed Decarboxylative Coupling Reactions of Carboxylic Acids and Their Derivatives
Yanhong Liu, Dongju Zhang
Prog Chem ›› 2025, Vol. 37 ›› Issue (2) : 281-292.
 PDF(9832 KB)
PDF(9832 KB)
 PDF(9832 KB)
PDF(9832 KB)
Research Process on Photoinduced Copper-Catalyzed Decarboxylative Coupling Reactions of Carboxylic Acids and Their Derivatives
The visible-light-driven copper-catalyzed decarboxylative coupling reaction of carboxylic acids and their derivatives is a novel, efficient, and green synthetic method. It allows the construction of various carbon-carbon and carbon-heteroatom bonds for the synthesis of a wide range of high-value-added chemicals, making it a hot topic in the field of modern synthetic chemistry. In recent years, researchers worldwide have conducted extensive studies in this area, achieving a series of innovative results that have been widely applied in organic synthesis, materials science, and medicinal chemistry. This paper reviews the latest experimental and theoretical advances in the visible-light-driven copper-catalyzed decarboxylative coupling reactions of carboxylic acids and their derivatives, with a focus on several typical cross-coupling reactions that form C—X (X = C, N, O, S) bonds. It also discusses the future development prospects of this catalytic method.
1 Introduction
2 Mechanism of photocatalyst and copper complex co-catalysis
3 Photocatalyst and copper complex co-catalyzed carboxylic acid (ester) decarboxylative coupling reactions
3.1 C—C coupling
3.2 C—N coupling
3.3 C—O coupling
3.4 C—S coupling
4 Conclusion and outlook
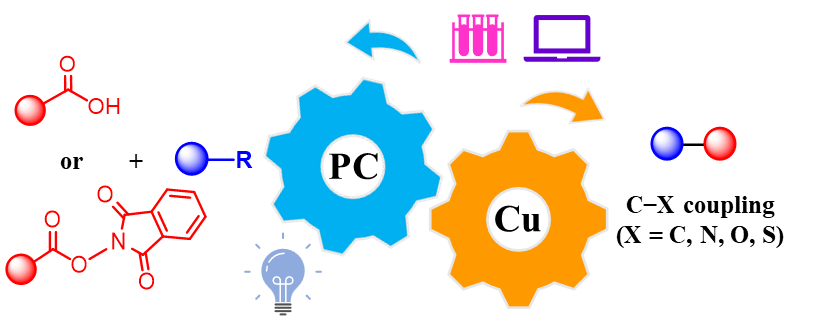
photocatalysis / copper complex / carboxylic acid / decarboxylation / cross-coupling reaction
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
Acetylene Chemistry: Chemistry, Biology and Material Science. Eds.: Diederich F, Stang P, Tykwinski R. Wiley-VCH, 2004.
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
/
| 〈 |
|
〉 |