Abbreviation (ISO4): Prog Chem
Editor in chief: Jincai ZHAO


Degradation of Antibiotics Using ZVI/H2O2 Fenton-Like Technology
Baizhou Lu, Zhanqiang Fang
Prog Chem ›› 2025, Vol. 37 ›› Issue (3) : 411-424.
Degradation of Antibiotics Using ZVI/H2O2 Fenton-Like Technology
ZVI/H2O2 Fenton-like technology overcomes some problems existing in the traditional homogeneous Fenton reaction, and can effectively remove antibiotics in water, which has good application potential. However, the degradation efficiency and mineralization rate of antibiotics in water by ZVI/H2O2 technology alone need to be improved. Therefore, researchers have adopted different strengthening measures to improve the deconta mination efficiency of ZVI/H2O2 technology and its mineralization rate of pollutants. In this paper, the research of antibiotics removal in water by ZVI/H2O2 technology is statistically analyzed. The main strengthening measures of ZVI/H2O2 technology and their effects on the system are summarized. The degradation efficiency, mechanism, advantages and disadvantages of antibiotics in water by different strengthening measures combined with ZVI/H2O2 technology are described and analyzed. Finally, this paper looks forward to the future development of ZVI/H2O2 technology for the degradation of antibiotics in water, and puts forward relevant suggestions for further research work.
1 Introduction
2 Development status of ZVI/H2O2 technology for removing antibiotics in water at home and abroad
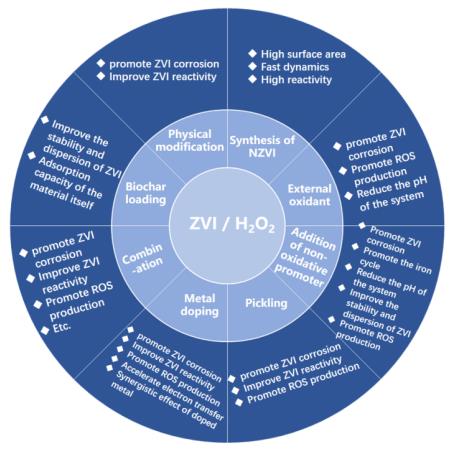
3 The main strengthening measures of ZVI/H2O2 technology and its effect on the system
3.1 Physical modification
3.2 Synthesis of n-ZVI
3.3 Biochar loading
3.4 External oxidant
3.5 Addition of non-oxidative promoter
3.6 Pickling
3.7 Metal doping
3.8 Other
3.9 combination
4 The degradation efficiency and mechanism of antibiotics in water by ZVI/H2O2 technology
5 Conclusion and outlook
zero-valent iron / H2O2 / Fenton-like / antibiotics / strengthening measures
| [1] |
|
| [2] |
|
| [3] |
(孙玉杰, 杨淑慎, 张琪, 武培, 魏娟. 山东化工, 2017, 46(16): 9.).
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(聂晓静, 大连理工大学硕士论文, 2017.).
|
| [23] |
(韩跃飞, 华东理工大学硕士论文, 2019.
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
(陈俊毅, 易云强, 方战强, 晏晓敏, 郑刘春. 华南师范大学学报(自然科学版), 2019, 51(1): 49.).
|
| [28] |
|
| [29] |
|
| [30] |
(万玲, 洪军, 刘保锋, 苏趋. 中国测试, 2015, 41(10): 44.).
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
(王炫栋, 伯绍毅, 季文浩, 王林拓, 李小忠, 陈寒松. 中国资源综合利用, 2020, 38(6): 4.).
|
| [47] |
|
| [48] |
(黄挺, 张光明, 张楠, 种珊, 刘毓璨, 朱佳. 环境工程学报, 2017, 11(11): 5892.).
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
(易云强, 方战强. 华南师范大学学报(自然科学版), 2018, 50(1): 28.).
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
(柴凡凡, 李克艳, 郭新闻. 应用化学, 2016, 33(02): 133.).
|
| [68] |
|
| [69] |
(方战强, 卢柏舟, 冼靖怡. 中国专利. CN111747504A. 2020.).
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
(邹亚辰, 贾小宁, 冉浪, 周林成, 赵泉林, 叶正芳. 化学反应工程与工艺, 2021, 37(2): 167.).
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
(王麒, 薛罡, 钱雅洁, 刘振鸿. 工业水处理, 2019, 39(9): 87.).
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
(杨波, 张永丽. 化学学报, 2019, 77(10): 1017.).
|
| [91] |
|
| [92] |
(李瑜辉, 谢武明, 吕文东, 黄子峻, 卞求实. 环境化学, 2022, 41(2): 707.).
|
| [93] |
|
| [94] |
|
| [95] |
(黄丹维, 何佳, 谷亚威, 何锋. 化学学报, 2017, 75(09): 866.).
|
| [96] |
|
| [97] |
|
| [98] |
|
/
| 〈 |
|
〉 |