 PDF(8593 KB)
PDF(8593 KB)


The Mechanisms of Homogeneous and Heterogeneous Reactions Involving Polyphenolic Compounds in the Water Treatment Process
Wuyuxin Zhu, Linjun Qin, Guorui Liu
Prog Chem ›› 2025, Vol. 37 ›› Issue (4) : 479-507.
 PDF(8593 KB)
PDF(8593 KB)
 PDF(8593 KB)
PDF(8593 KB)
The Mechanisms of Homogeneous and Heterogeneous Reactions Involving Polyphenolic Compounds in the Water Treatment Process
Polyphenolic compounds are a class of naturally occurring bioactive substances widely found in the environment. Their characteristics,such as low toxicity,low cost,and broad availability,make them become to be widely used chelating agents,reducing agents,and capping agents for treating typical pollutants in water. Currently,polyphenols are extensively used in advanced oxidation processes (AOPs) through the coupling of common transition metal ions and peroxides. However,the chemical mechanisms of polyphenolic substances in water pollution remediation still lack systematic conclusions. This study reviews and summarizes the compositions of homogeneous and heterogeneous systems containing polyphenolic compounds,as well as the pro-oxidant,antioxidant,and chelating-reduction effects exhibited by polyphenols within these systems. It explains the main active species generated by polyphenolic substances under different systems from both radical and non-radical perspectives,along with the corresponding mechanisms for the removal of water pollutants. The dual role of polyphenols as natural redox mediators (RMs) in constructing complex catalytic systems is emphasized,and the effects of external energies such as light,heat,electricity,ultrasound,and plasma on the reaction mechanisms and pollutant degradation effectiveness in these systems are described. Finally,the article looks ahead to the future development directions of polyphenolic compounds in the field of water treatment.
Contents
1 Introduction
2 H2O2/PS/PAA activation
2.1 ROS of H2O2/PS/PAA
2.2 Polyphenols/Fe(Cu) ions/peroxide systems
2.3 Chelation and reduction of polyphenol-metal ions
2.4 Non-radical reactions
3 High-valent metal species
3.1 Fe ions
3.2 Cu ions
3.3 Mn ions
4 Solid catalyst
4.1 Zero-valent metal monomers
4.2 Monometallic compounds
4.3 Polymetallic compounds
4.4 Metal-organic complexes
4.5 Carbon-based materials
4.6 Inorganic salt supported metal catalysts
5 Polyphenol-SQ•--Quinone
5.1 Periodate and permanganate
5.2 Peroxide
5.3 O2,H2O and others
5.4 Redox mediators
6 External energy
7 Conclusion and outlook
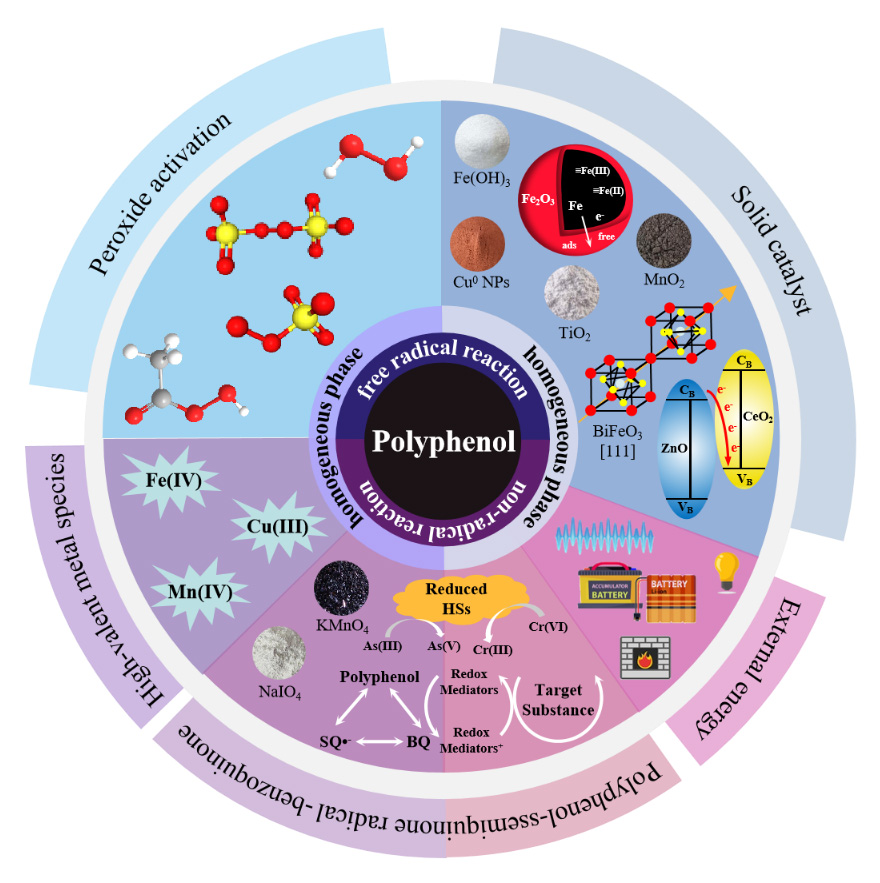
polyphenols / free radical chemistry / pro-oxidation / heterogeneous reactions / redox mediators (RMs) / advanced oxidation processes (AOPs)
| [1] |
|
| [2] |
|
| [3] |
Belščak Cvitanović A,
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
|
| [183] |
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [194] |
|
| [195] |
|
| [196] |
|
| [197] |
|
| [198] |
|
| [199] |
|
| [200] |
|
| [201] |
|
| [202] |
|
| [203] |
|
| [204] |
|
| [205] |
|
| [206] |
|
| [207] |
|
| [208] |
|
| [209] |
|
| [210] |
|
| [211] |
|
| [212] |
|
| [213] |
|
| [214] |
|
| [215] |
|
| [216] |
|
| [217] |
|
| [218] |
|
| [219] |
|
| [220] |
|
| [221] |
|
| [222] |
|
| [223] |
|
| [224] |
|
| [225] |
|
| [226] |
|
| [227] |
|
| [228] |
|
| [229] |
|
| [230] |
|
| [231] |
|
| [232] |
|
| [233] |
|
| [234] |
|
| [235] |
|
| [236] |
|
| [237] |
|
| [238] |
|
| [239] |
|
| [240] |
|
| [241] |
|
| [242] |
|
| [243] |
|
| [244] |
|
| [245] |
|
| [246] |
|
| [247] |
|
| [248] |
|
| [249] |
|
| [250] |
|
| [251] |
|
| [252] |
|
| [253] |
|
| [254] |
|
| [255] |
|
| [256] |
|
| [257] |
|
| [258] |
|
/
| 〈 |
|
〉 |