 PDF(8103 KB)
PDF(8103 KB)


Post-Combustion CO2 Capture Materials
Jiajia Jiang, Junhu Zhao, Qin Yu, Tian Zhang
Prog Chem ›› 2025, Vol. 37 ›› Issue (4) : 593-611.
 PDF(8103 KB)
PDF(8103 KB)
 PDF(8103 KB)
PDF(8103 KB)
Post-Combustion CO2 Capture Materials
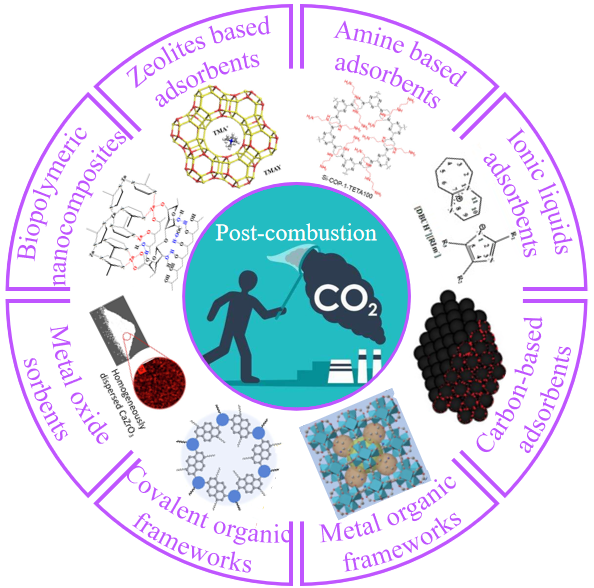
The sustained development of industry has brought enormous economic benefits,but it has also caused great harm to the environment. The excessive CO2 emissions from fossil fuel combustion are released into the natural environment,posing a threat to the environment and human health. So people are working hard to develop materials that can effectively capture CO2. At present,CO2 capture mainly occurs after the combustion of fossil fuels. According to the design standards for CO2 adsorbents,a variety of CO2 capture materials have been designed and developed,including solid adsorbents,liquid adsorbents,and multiphase adsorbents. The adsorption mechanisms of various adsorbents are also different,including adsorption,absorption,or a combination of both mechanisms. This review focuses on the capture performance,absorption mechanism,advantages and disadvantages of various common types of current adsorbents,and introduces amine solution absorbents,zeolite-based adsorbents,ionic liquids-based adsorbents,carbon-based adsorbents,metal-organic framework materials,covalent organic framework materials,metal-oxide materials,and biopolymer nanocomposites,respectively,with an outlook of the future development of CO2 adsorbent materials.
Contents
1 Introduction
1.1 Current status and hazards of CO2 emissions
1.2 CO2 capture technology
1.3 Criteria for designing CO2 capture materials
2 CO2 capture materials
2.1 Amine solution absorbents
2.2 Zeolites based adsorbents
2.3 Ionic liquids absorbents
2.4 Carbon-based adsorbents
2.5 Metal organic framworks
2.6 Covalent organic frameworks
2.7 Metal oxide sorbents
2.8 Biopolymeric nanocomposites
3 Comparison and Prospect of Capture Materials
4 Conclusion
CO2 concentration / post-combustion CO2 / capture / adsorption / capture materials / multiphase adsorbents
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
/
| 〈 |
|
〉 |