

Proton Exchange Membranes Based on All-Carbon Backbone Aromatic Polymers
Received date: 2023-05-15
Revised date: 2023-06-24
Online published: 2023-08-07
Supported by
National Natural Science Foundation of China(92261110)
National Natural Science Foundation of China(22075097)
Proton exchange membranes are widely used in energy storage and conversion technologies such as fuel cells, redox flow batteries, and water electrolysis, which are key materials urgently needed under the “dual carbon” goal. Perfluorosulfonic acid membranes show high proton conductivity and mechanical properties, which are currently the most widely used proton exchange membranes materials. However, these membranes suffer from the following disadvantages, such as greatly decreased proton conductivity at low humidity conditions, low glass transition temperature, and complex synthesis process. In the past decades, efforts have been devoted to the development of various alternative materials, such as polyether ether ketone, polyphenylene oxide, polysulfone and polyimide. However, the main chains of these polymers usually contain heteroatoms. Upon working in a complex practical condition for a long time, the heteroatom position is prone to break, which reduces the chemical stability of these materials. In contrast, the all-carbon backbone aromatic polymers have excellent chemical stability, thermal stability, and mechanical properties, and are a class of potential alternative materials that have attracted extensive attention in recent years. In this review paper, we summarize the recent research progress of all-carbon backbone aromatic polymers, focusing on the synthesis strategies, structure-performance relationships, as well as the applications of these polymers in proton exchange membranes.
1 Introduction
2 Proton exchange membranes based on polyphenylenes
2.1 Synthesis and general properties
2.2 Straight-chain sulfonated polyphenylene proton exchange membranes
2.3 Curve-chain sulfonated polyphenylene proton exchange membranes
3 Proton exchange membranes based on phenylated polyphenylenes
3.1 Synthesis and general properties
3.2 Sulfonated phenylated polyphenylene proton exchange membranes
3.3 Phosphoric acid doped phenylated polyphenylene proton exchange membranes
4 Proton exchange membranes based on poly(arylene-alkane)s
4.1 Synthesis and general properties
4.2 Sulfonated poly(arylene-alkane)proton exchange membranes
4.3 Phosphorylated poly(arylene-alkane)proton exchange membranes
4.4 Phosphoric acid doped poly(arylene-alkane)proton exchange membranes
5 Conclusion and outlook

Li Tingting , Li Haibin , Liu Binghui , Zhao Chengji , Li Haolong . Proton Exchange Membranes Based on All-Carbon Backbone Aromatic Polymers[J]. Progress in Chemistry, 2023 , 35(11) : 1559 -1578 . DOI: 10.7536/PC230513
表1 聚苯、苯基聚苯和聚芳烷的典型结构和合成方法Table 1 Typical structures and synthesis methods of polyphenylenes, phenylated polyphenylenes and poly(arylene-alkane)s |
图3 部分聚苯的化学结构:(a) PBPDSA, (b) PPDSA, (c) BXPY, (d) PPDSA-g-DDB3-24%, PPDSA-g-OcB11-16%和B20P80-g-OcB7-18%, (e) B20P80-g-BP10%-210C-3h[44,47,48]Fig.3 Chemical structures of some polyphenylenes: (a) PBPDSA, (b) PPDSA, (c) BXPY, (d) PPDSA-g-DDB3-24%, PPDSA-g-OcB11-16% and B20P80-g-OcB7-18%, (e) B20P80-g-BP10%-210C-3h[44,47,48] |
图9 部分苯基聚苯的化学结构: (a) sPPB-H+, (b) sPPN-H+, (c) sPPB (x% DB)-H+, (d) sTPPyPP-H+[66, 70~72]Fig.9 Chemical structure of some phenylated polyphenylenes: (a) sPPB-H+, (b) sPPN-H+, (c) sPPB (x% DB)-H+, (d) sTPPyPP-H+[66,70~72] |
表2 部分苯基聚苯的杨氏模量、断裂强度、断裂伸长率、热分解温度和芬顿试剂测试Table 2 Young’s modulus, tensile strength, elongation at break, thermal decomposition temperature and Fenton’s reagent test of some phenylated polyphenylenes |
| Polymer | Young’s modulus(MPa) | Tensile strength (MPa) | Elongation at break (%) | Td(℃) | Fenton’s reagent testa) | ref | |
|---|---|---|---|---|---|---|---|
| sPPP-H+ | 1059 | 43.5 | 29.1 | — | Dissolve after 3 h | 65 | |
| sPPB-H+ | 1331 | 59.6 | 17.5 | 260 | Intact after 1 h | 66 | |
| sPPN-H+ | 1170 | 53.3 | 18.7 | 260 | Intact after 1 h | 66 | |
| sPPP (m)-H+ | 1008~1407 | 39.6~51.3 | 15.9~31.7 | — | Broken after 3 h | 67 | |
| sPPB (x% DB)-H+ | 1267~1616 | 52.8~62.1 | 12.9~26.3 | 280 | Intact after 1 h | 70 | |
| sTPPPP-H+ | 584.1 | 54.8 | 36.8 | 246 | Completely dissolved after 5.3 h | 71 | |
| sTPPyPP-H+ | 401.8 | 43.3 | 55.5 | 326 | There are still residues after 6.3 h | 71 |
a) The concentration of Fenton’s reagent is 3% H2O2, 2 ppm Fe2+,the test temperature is 80℃ |
表3 部分聚芳烷质子交换膜的芬顿试剂测试Table 3 Fenton’s reagent test of some poly(arylene-alkane) proton exchange membranes |
| Structures | Fenton’s reagent | Temperature(℃) | Test Results | ref |
|---|---|---|---|---|
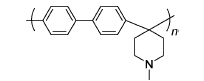 PBP | 3% H2O2,3 ppm Fe2+ | 68 | Broken after 26 h | 111 |
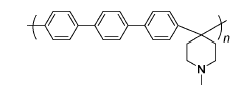 PTP | 3% H2O2,3 ppm Fe2+ | 68 | Broken after 200 h | 111 |
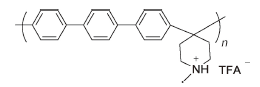 PTP-TFA | 3% H2O2,4 ppm Fe2+ | 68 | Mass loss of 17% after 84 h | 112 |
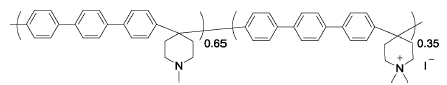 PTP-35%Me | 3% H2O2,4 ppm Fe2+ | 68 | Mass loss of 20% after 84 h | 112 |
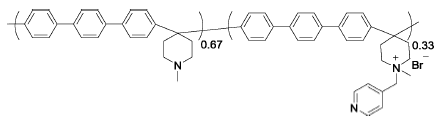 PTP-33%Py | 3% H2O2,4 ppm Fe2+ | 68 | Mass loss of 30% after 84 h | 112 |
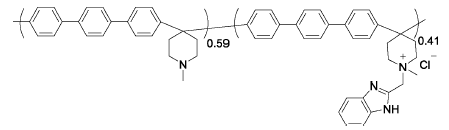 PTP-41%BeIm | 3% H2O2,4 ppm Fe2+ | 68 | Mass loss of 35% after 84 h | 112 |
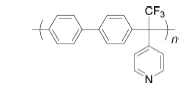 PBAP | 3% H2O2,4 ppm Fe2+ | 68 | Broken after 75 h | 113 |
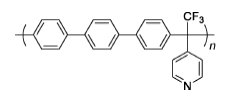 PTAP | 3%H2O2,4 ppm Fe2+ | 68 | Polymer membrane remains intact after 450 h | 113 |
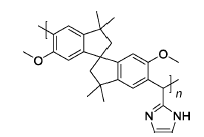 2-IMPIM | 3% H2O2,2 ppm Fe2+ | 80 | Mass loss of 19% at 50 h | 116 |
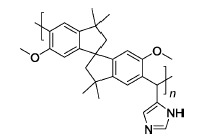 4-IMPIM | 3% H2O2,2 ppm Fe2+ | 80 | Mass loss of 13% for 50 h | 116 |
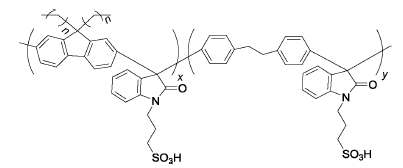 MFxDPy-PS、HFxDPy-PS | 3% H2O2,4 ppm Fe2+ | 80 | All polymer membranes remain intact after 10 days | 95 |
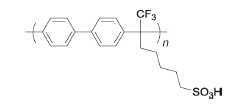 BPSA | 3% H2O2,2 ppm Fe2+ | 80 | Mass loss of 2% for 1 h | 98 |
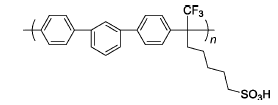 m-TPSA | 3% H2O2,2 ppm Fe2+ | 80 | Mass loss of 1% for 1 h | 98 |
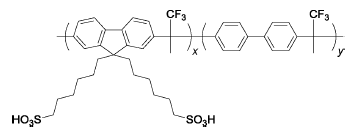 Poly(FLx-BPy)-SO3H | — | 80 | The mass loss of all polymer membranes was less than 1% after 4 h | 99 |
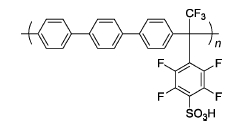 | 3% H2O2,2 ppm Fe2+ | 80 | Broken after 24 h | 100 |
| SP100 | ||||
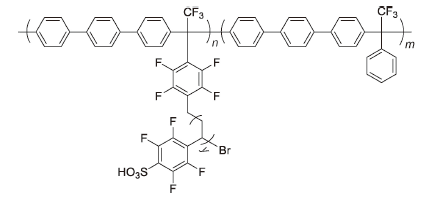 SMn-X-IEC | 3% H2O2,2 ppm Fe2+ | 80 | The signal peak of side chain fluorine decreased about 70% after 1 h | 101 |
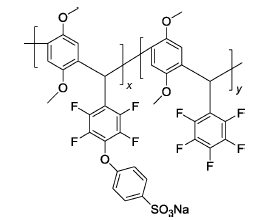 SP1 | 3% H2O2,2 ppm Fe2+ | 80 | Broken after 6 h | 90 |
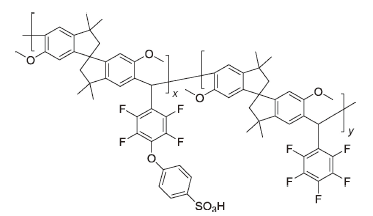 SP2 | 3% H2O2,2 ppm Fe2+ | 80 | Broken after 8.5 h | 90 |
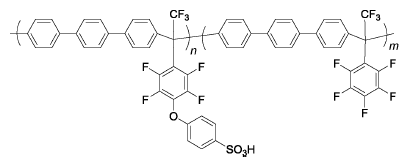 SP3 | 3% H2O2,2 ppm Fe2+ | 80 | Broken after 10.5 h | 90 |
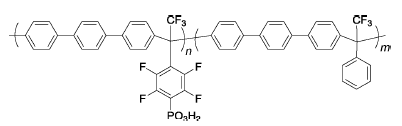 PPx | 3% H2O2,2 ppm Fe2+ | 80 | PP55, PP72, PP83 membranes become opaque after 11 days | 118 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
/
| 〈 |
|
〉 |