

Construction and Application in Food Contaminants Detection of Novel Optical Fiber Biosensors
Received date: 2023-04-24
Revised date: 2023-08-30
Online published: 2023-12-20
Supported by
National Natural Science Foundation of China(22276080)
Shuangchuang Ph.D Award of Jiangsu Province(1184902001)
Food safety is closely related to people’s quality of life. The establishment of simple, sensitive and intelligent detection methods for food contaminants is an important guarantee for food safety and health management. Nevertheless, traditional analysis methods have certain limitations such as time-consuming detection process, high cost, and complicated operation. Optical fiber biosensors, which rely on the interaction between light and fluids, have the characteristics of good signal sensitivity, rapid detection and real-time response. They have recently emerged as advanced optical sensing methods with diverse functions and high sensitivity, and can realize rapid and accurate detection of various pollutants in food. In this review, we summarized the basic principles, classification and research status of various novel optical fiber biosensors. The applications in the detection of various pollutants such as mycotoxins, heavy metal ions, antibiotics, and pesticide residues in food samples were introduced. Furthermore, the development trend of this novel sensing strategy was also briefly discussed.
1 Introduction
2 Optical fiber biosensor
2.1 Composition of optical fiber biosensor
2.2 Basic principle of optical fiber biosensor
2.3 Classification of optical fiber biosensor
3 Application of optical fiber biosensors in detection of food contaminants
3.1 Mycotoxin
3.2 Heavy metal ion
3.3 Antibiotic
3.4 Pesticide residue
3.5 Pathogen
4 Conclusion and prospect
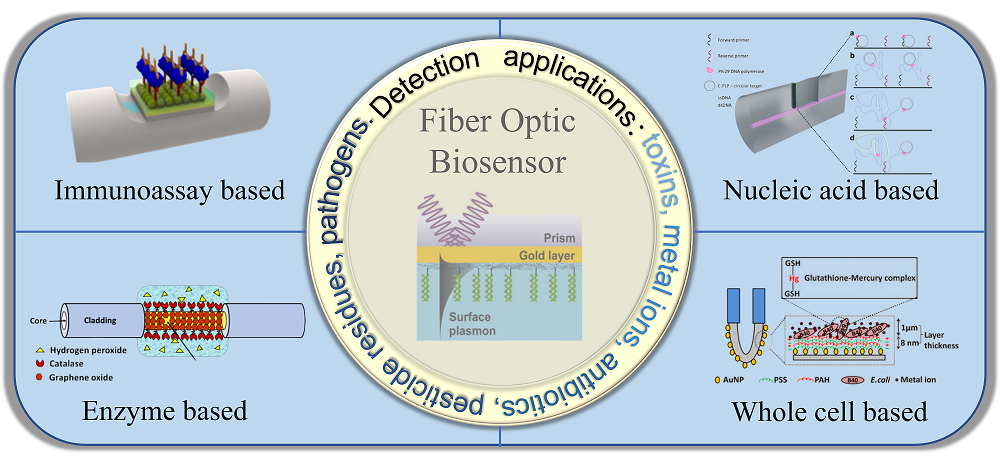
Key words: optical fiber; biosensor; food contaminant; highly sensitive detection
Jialin Huang , Yaohua Qin , Sheng Tang , Dezhao Kong , Chang Liu . Construction and Application in Food Contaminants Detection of Novel Optical Fiber Biosensors[J]. Progress in Chemistry, 2024 , 36(1) : 120 -131 . DOI: 10.7536/PC230423
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
/
| 〈 |
|
〉 |