

Synthesis of Two-Dimensional Layered Zeolites and Their Catalysis, Adsorption and Separation Applications
Received date: 2023-07-18
Revised date: 2023-11-21
Online published: 2024-02-26
Supported by
National Key R&D Program of China(2018YFA0209402)
National Natural Science Foundation of China(22072028)
National Natural Science Foundation of China(22088101)
Shanghai Natural Science Foundation(22ZR1407200)
Compared with three-dimensional zeolites, two-dimensional layered zeolites have greater advantages in many fields, with larger surface area, shorter diffusion distance and more ductile structure. In recent years, the research on two-dimensional layered zeolites has become a new hotspot. Based on previous research and summary, this article summarizes the synthesis methods of two-dimensional zeolites in the past five years from two types of synthesis perspectives (bottom-up and top-down methods), with a focus on reviewing the progress of different synthesis methods for the same topology of zeolite. In addition, this article briefly describes the applications of two-dimensional zeolites in the fields of catalysis, adsorption, and separation and looks forward to the broad application prospects of two-dimensional zeolites so as to provide theoretical guidance and reference basis for the synthesis and application of two-dimensional zeolites.
1 Introduction
2 Synthesis of two-dimensional layered zeolites
2.1 Bottom-up synthesis method
2.2 Top-down synthesis method
3 Application of two-dimensional layered zeolite
3.1 Catalysis
3.2 Adsorption
3.3 Separation membrane
4 Conclusion and outlook
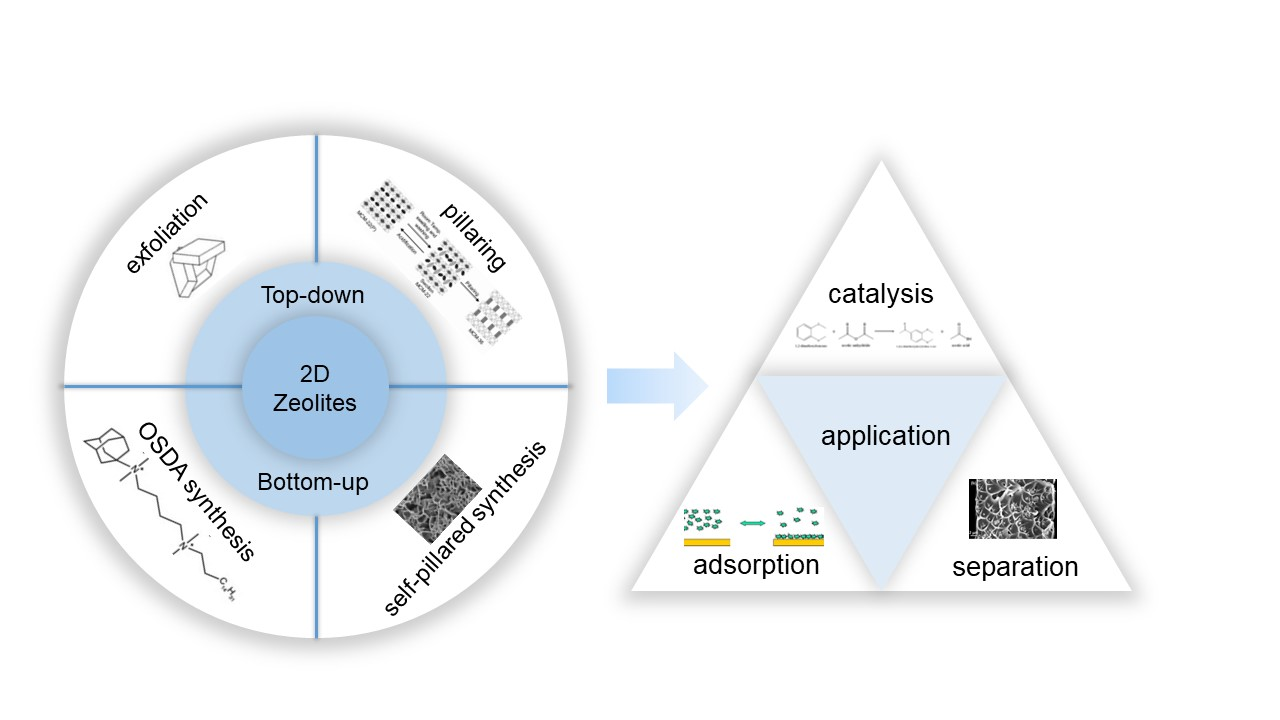
Key words: 2D zeolite; synthesis; acid catalysis; adsorption; separation membrane
Shiyu Hu , Yueer Yan , Yahong Zhang , Zhendong Wang , Yi Tang . Synthesis of Two-Dimensional Layered Zeolites and Their Catalysis, Adsorption and Separation Applications[J]. Progress in Chemistry, 2024 , 36(3) : 319 -334 . DOI: 10.7536/PC230716
图4 用C22-6-6/TPAOH摩尔比为10/0(a)、10/1(b)、10/2(c)、10/3(d)、10/5(e)、10/8(f)、10/12(g)和10/20(h)制备的MFI沸石的扫描电子显微镜(SEM)图像[44]。Si/Al比为~40(i)的商用MFI沸石作为对照Fig. 4 SEM images of MFI zeolites obtained with C22-6-6/TPAOH molar ratio of (a) 10/0, (b) 10/1, (c) 10/2, (d) 10/3, (e) 10/5, (f) 10/8, (g) 10/12, and (h) 10/20, respectively, in the dual template synthesis. (i) Commercial MFI with Si/Al ratio of ∼40 was used for comparison[44]. Copyright © 2014, American Chemical Society |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
/
| 〈 |
|
〉 |