

Catalytic Conversion of Hydroxyl Compounds : Conversion of Phenols and Alcohols to Ethers and Esters
Received date: 2023-07-17
Revised date: 2023-09-28
Online published: 2024-01-10
Supported by
Fundamental Research Program of Shanxi Province(20210302124472)
National Natural Science Foundation of China(22072176)
Shanxi Science and Technology Department(20210302123012)
Shanxi Science and Technology Department(201801D221093)
Shanxi Science and Technology Department(202203021211003)
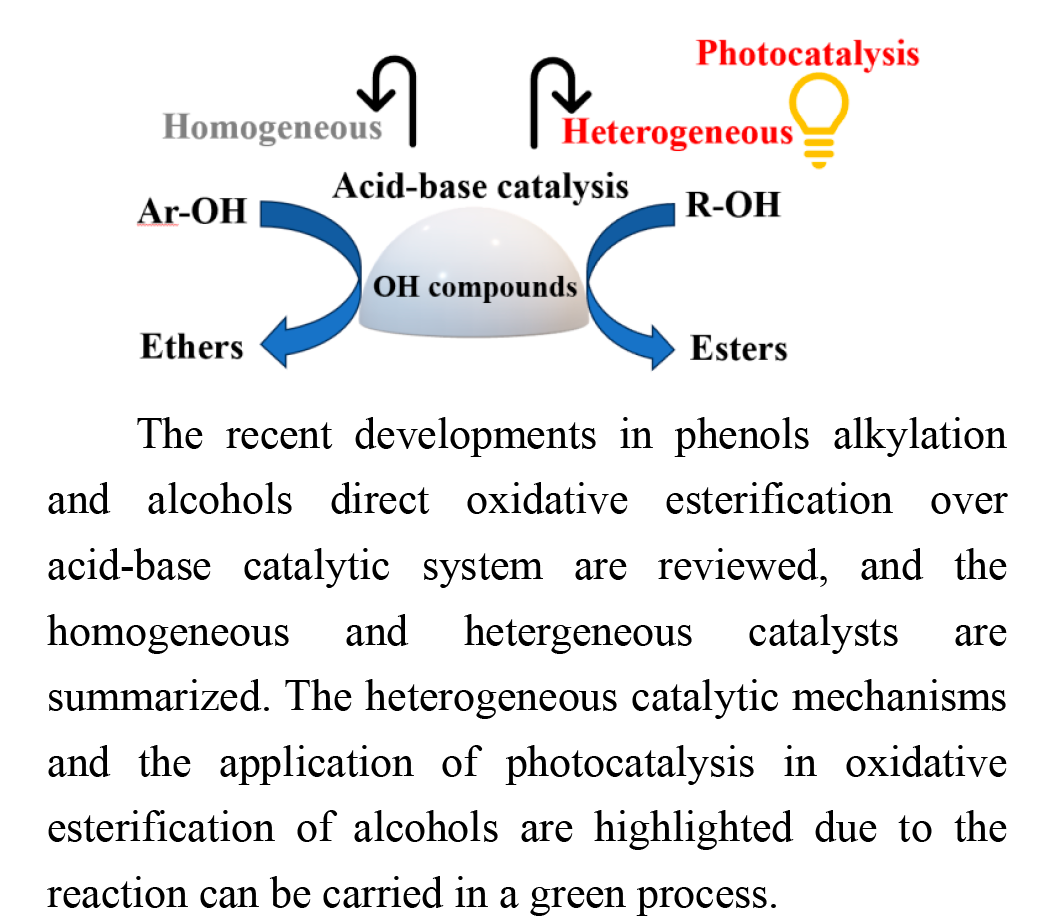
With the background of rapid economic development, the green synthesis of high-value-added chemicals has attracted great interest. Ethers and Esters, the products of hydroxyl compound conversion, are important green chemical products. However, the harsh reaction conditions limit their application. Herein, we review the developments in the catalysis of phenols alkylation to ethers and alcohols oxidative esterification to esters. The modification strategy and catalytic mechanism of the catalytic systems are summarized. The heterogeneous catalytic system and its mechanisms have been mainly discussed. It is found that the acid-base synergistic catalysis and the synergistic effect between metal and support are favorable for the green synthesis of ethers and esters under mild reaction condition. Besides, the application of photocatalysis in oxidative esterification of alcohols is highlighted because the photocatalytic reaction is considered a promising green synthesis method. Finally, the research on the catalytic conversion of hydroxyl compounds are summarized and prospected, and we believe that the synthesis and modification of new catalysts and the exploration of catalytic mechanisms is still a promising research field.
1 Introduction
2 Activation of phenols hydroxyl group:alkylation of phenols
2.1 Homogeneous catalyst
2.2 Heterogeneous catalyst
2.3 Alkylating agent
2.4 Catalytic mechanism of phenols alkylation
3 Activation of alcohols hydroxyl group:oxidative esterification of alcohols
3.1 Homogeneous catalyst
3.2 Heterogeneous catalyst and catalytic mechanism
4 Photocatalytic oxidative esterification of alcohols
5 Conclusions and outlook
Xiaoyu Wang , Ruiyi Wang , Xiangpeng Kong , Yulan Niu , Zhanfeng Zheng . Catalytic Conversion of Hydroxyl Compounds : Conversion of Phenols and Alcohols to Ethers and Esters[J]. Progress in Chemistry, 2024 , 36(3) : 335 -356 . DOI: 10.7536/PC230714
表1 不同烷基化剂在酚烷基化反应中性能的比较Table 1 Activity comparison of different alkylating agents used in the alkylation of phenols |
| Phenols | Alkylating agent | Conv. (%) a) | Sel. (%) b) | Ref |
|---|---|---|---|---|
| catechol | methanol | 14 | 42.3 | 16 |
| DMC c) | 94 | 79.1 | ||
| phenol | DEC d) | 96.1 | 98.3 | 23 |
| ethanol | 9.3 | - | ||
| phenol | DMC | 100 | 95 | 24 |
| phenol | DEC | 95 | 85 | 25 |
a). conversion of phenols, b). selectivity of O-alkylated product, c). dimethyl carbonate, d). diethyl carbonate |
图2 多种分子筛催化间甲酚与甲醇烷基化反应的催化性能研究(反应温度:523 K)Fig. 2 Catalytic results for the alkylation of m-cresol with methanol over various molecular sieves, reaction temperature: 523 K |
图5 苯酚分子的苯环以水平方式吸附于催化剂表面的酸性位点Fig. 5 Parallel orientation of the aromatic ring of phenol molecule on the acidic site of catalyst surface |
图6 苯酚分子的苯环以垂直方式吸附于催化剂表面的酸性位点Fig. 6 Vertical orientation of the aromatic ring of phenol molecule on the acidic site of catalyst surface |
表2 不同均相催化剂在苯甲醇与甲醇氧化酯化反应中性能的比较Table 2 Comparison of different homogeneous catalysts activity in the oxidative esterification of benzyl alcohol with methanol |
| Catalysts | Ligands | Additives | Temp. (K) | Time (h) | Yield (%) a) | Ref |
|---|---|---|---|---|---|---|
| [PdCl2 (CH3CN)2] | - | NaOtBu | 318 | 12 | 74 | 33 |
| [Pd (OAc)2] | - | Na2CO3 | 313 | 24 | 99 | 34 |
| [Pd (OAc)2] | b) | K2CO3 | 333 | 24 | 88 | 35 |
| NaAuCl4·H2O | c) | K2CO3 | 353 | 5 | 97 | 36 |
| [Ru(p-cymene)Cl2]2 | - | CsCO3 | 403 | 24 | 90 | 37 |
| [Ru (PPh3)4H2] | d) | - | 383 | 48 | 83 | 38 |
| Ru complex | - | KOH | 388 | 72 | 99.5 | 39 |
| ZnBr2 | - | H2O2 | 303 | 16 | 30 | 41 |
a) Yield of methyl benzoate; b) n-butyl bis (1-adamantyl) phosphine; c) N-heterocyclic carbenes salt; d) 4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene |
图11 Co3O4-N@C催化苯甲醇与甲醇的氧化酯化反应方程式Fig. 11 Reaction formulas of the oxidative esterification of benzyl alcohol with methanol on the Co3O4-N@C catalyst |
图12 不添加碱的条件下,Co@CN(800)催化对硝基苯甲醇与甲醇的氧化酯化反应示意图[49]Fig. 12 Schematic illustration of the oxidative esterification of p-nitrobenzyl alcohol with methanol on Co@CN(800) catalyst without the addition of base. Reprinted (adapted) with permission from ref 49. Copyright (2015) American Chemical Society |
图13 PdAu合金催化乙醇的自酯化反应示意图[50]Fig. 13 Self-esterification of ethanol over PdAu alloy catalyst. Reproduced with permission from. Reprinted (adapted) with permission from ref 50. Copyright (2019) American Chemical Society |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
/
| 〈 |
|
〉 |