

Triptycene Based Electroluminescent Materials
Received date: 2023-09-28
Revised date: 2023-10-27
Online published: 2024-01-20
Supported by
National Natural Science Foundation of China(21572001)
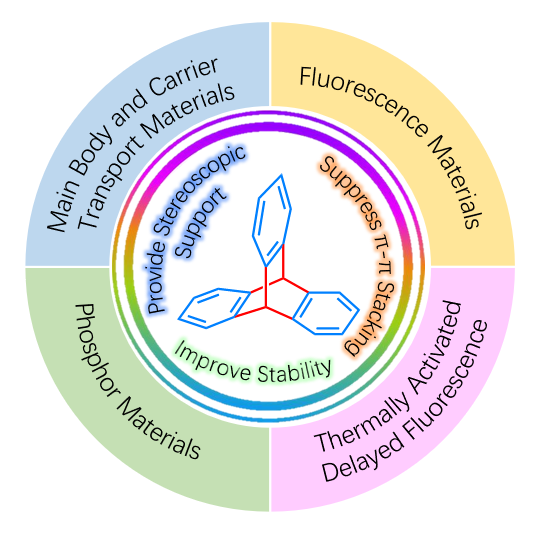
Organic light-emitting diodes (OLEDs) have the advantages of self-luminous, high efficiency, light and thin structure, and can achieve diverse designs such as transparency and flexibility. They have broad application prospects in fields such as display and lighting. Triptycene is a stable, three-dimensional, and rigid structure formed by connecting three benzene rings through saturated carbon, and the conjugation between the three benzene rings is very small. Different substituents on the three benzene rings can also achieve very stable chirality. The triptycene group can provide an ideal rigid three-dimensional framework for the design of high-performance luminescent materials, in order to enhance the stability of luminescent materials, regulate intermolecular interactions (reduce concentration quenching while improving film formation), and maintain a stable chiral environment. In this paper, the research progress of incorporating triptycene group into electroluminescent electron transport layer and light-emitting layer material molecules is reviewed. The future of triptycene based electroluminescent materials is also prospected. By analyzing and summarizing the influence of triptycene group on material properties, its advantages are identified, so as to play a role in attracting more researchers to carry forward the advantages of triptycene in the field of new materials in the future.
1 Introduction
2 The host materials and electron transport materials with triptycene group
3 Fluorescent materials with triptycene group
4 TADF materials with triptycene group
5 Iridium complex phosphorescent materials with triptycene group
Huihui Xu , Qingsong Wang , Junjie Mao , Bihai Tong , Qianfeng Zhang . Triptycene Based Electroluminescent Materials[J]. Progress in Chemistry, 2024 , 36(3) : 393 -400 . DOI: 10.7536/PC230917
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
(a)
(b)
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(王英杰, 潘泽晖, 陶正煜, 王妍, 童碧海, 冯敏强, 宋明星. 高等学校化学学报, 2023, 44(4): 20220562.)
|
| [23] |
|
| [24] |
(a)
(b)
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
/
| 〈 |
|
〉 |