

Synthesis of Multi-Cyclic Hydrocarbon High-Density Aviation Fuels from Biomass
Received date: 2023-07-17
Revised date: 2023-09-18
Online published: 2024-02-26
High-density aviation fuels are a type of hydrocarbon which are synthesized to improve the flight performance of aerospace vehicles. They have the advantages of high density, high volumetric net heat of combustion value, and can effectively improve the flight performance of vehicles such as range, speed, load, etc. With the decrease of global fossil resources and the continuous deterioration of the ecological environment, the synthesis of high-density aviation fuels from biomass has become a research hotspot. In this review, the research progress in synthesis of multi-cyclic hydrocarbon high-density aviation fuels from platform molecules and derivatives in recent years is discussed. The common C-C bond coupling methods for constructing the multi-cyclic structure are introduced, including aldol condensation reaction, alkylation reaction, aldol-hydrodeoxygenation-alkylation reaction, Diels-Alder reaction, photoinduced 2+2 cycloaddition, rearrangement reaction. The new progress in synthesis of petroleum based high-density aviation fuels or multi-cyclic hydrocarbon mixed fuels from platform molecules is listed. The properties of a large number of multi-cyclic hydrocarbon high-density aviation fuels are summarized, and the influence of molecular structure and composition on fuel properties are discussed. Introduction of the appropriate substituent groups and synthesis of multi-component fuels are the main methods to improve the comprehensive properties of fuels. Synthesis of petroleum-based high-density fuels using platform molecules is another strategy to improve the properties of fuels. Finally, the development trend of synthesis of multi-cyclic hydrocarbon high-density aviation fuels using platform molecules from biomass is prospected.
1 Introduction
2 Aldol condensation reaction
3 Alkylation and Aldol-Hydrodeoxygenation-Alkylation reaction
4 Diels-Alder reaction
5 Photoinduced 2+2 cycloaddition reaction
6 Rearrangement reaction
7 Summary of fuel properties
8 Conclusion and outlook

Chongya Kong , Fangfang Tan , Yizhuo Wang , Hong Wang , Zhanchao Li . Synthesis of Multi-Cyclic Hydrocarbon High-Density Aviation Fuels from Biomass[J]. Progress in Chemistry, 2024 , 36(3) : 448 -462 . DOI: 10.7536/PC230713
表1 由生物质原料合成的多环碳氢高密度航空燃料性能总结Table 1 Properties of multi-cyclic hydrocarbon high-density aviation fuels from biomass |
| Feedstock | Main component structure | Density (20 ℃, g/mL) | Freezing point (℃) | Heat value (MJ/L) | Viscosity (25 ℃, mm2/s) | Ref |
|---|---|---|---|---|---|---|
| Cyclopentanone | 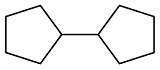 | 0.866 | -38 | 36.7 | 1.62 | 23 |
| Cyclopentanone and n-propanol | 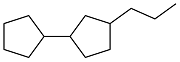 | 0.854 | < -80 | 38.12 | 2.294 (20 ℃) | 21 |
| Cyclopentanone and benzyl alcohol | 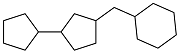 | 0.906 | -58 | 39.42 | 9.923 (20 ℃) | 21 |
| Cyclopentanone | 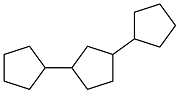 | 0.91 | — | — | 4.774 | 24 |
| Cyclopentanone | 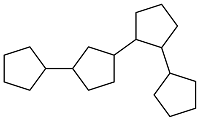 | 0.943 | -39.5 | — | — | 25 |
| 2, 5-hexanedione | 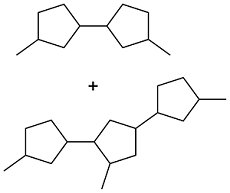 | 0.88 | -48 | — | — | 29 |
| Linalool or 5-methylfurfural | 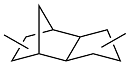 | 0.94 | < -40 | 39.0 | 60 (-40 ℃) | 46,48 |
| Furfuryl alcohol or xylose | 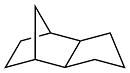 | 0.94 | -79 | 39.6 | 19 (-40 ℃) | 47 |
| Cyclopentanone | 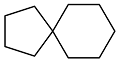 | 0.87 | -76 | 37.16 | 2.12 | 59 |
| Cyclopentanone and cyclopentadiene | 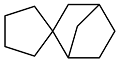 | 0.952 | -53 | 40.18 | 5.9 | 44 |
| Cyclohexanone | 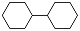 | 0.887 | 1.2 | 38.11 | 3.72 | 63 |
| Cyclohexanone and dimedone | 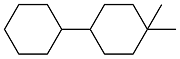 | 0.87 | -26 | 37.81 | 6.589 (20 ℃) | 18 |
| Isophorone | 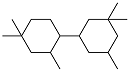 | 0.858 | -51 | — | — | 31 |
| Cyclohexanone | 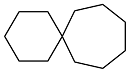 | 0.893 | -51 | 38.41 | 4.37 | 59 |
| Dimedone and cyclohexenone | 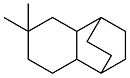 | 0.921 | -20 | 39.63 | 8.624 (20 ℃) | 18 |
| 2-benzylphenol | 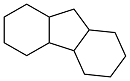 | 0.959 | -15 | 40.1 | 1752 (20 ℃) | 38 |
| 4-methylbenzaldehyde and cyclohexanone | 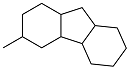 | 0.99 | -22 | — | — | 39 |
| 2-methylbenzaldehyde and cyclohexanone | 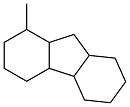 | 0.96 | -3 | — | — | 39 |
| Cyclopentanol | 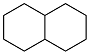 | 0.896 | -37 | — | — | 60 |
| Cyclohexanol and methylcyclopentane | 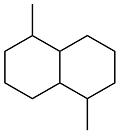 | 0.88 | < -51 | 37 | 22 (-40 ℃) | 61 |
| Isophorone and cyclohexene | 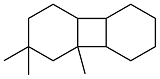 | 0.903 | -55 | 38.77 | 7.2 | 57 |
| Isophorone | 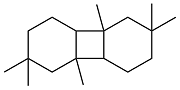 | 0.892 | -40 | 38.58 | 22.4 | 57 |
| Isophorone and β-pinenes | 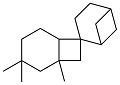 | 0.911 | -51 | 38.67 | — | 58 |
| 2-benzylphenol | 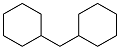 | 0.876 | -20 | 36.96 | 5.1 (20 ℃) | 38 |
| Benzylalcohol and 4-ethylphenol | 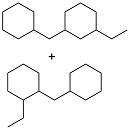 | 0.873 | -42 | 37.27 | 10.7 (20 ℃) | 34 |
| Dimedone, benzaldehyde and acetone | 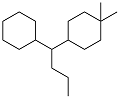 | 0.883 | -70 | 38.51 | 49.47 (20 ℃) | 18 |
| Dimedone and 5-methylfurfural | 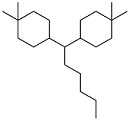 | — | -55 | 43.4 MJ/kg | — | 17 |
| 2-methylbenzaldehyde and t-butyl methyl ketone | 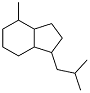 | 0.895 | -43 | 36.96 | 5.1 (20 ℃) | 42 |
| 4-ethylbenzaldehyde and t-butyl methyl ketone | 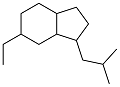 | 0.902 | -50 | 37.27 | 10.7 (20 ℃) | 42 |
| 2-methylbenzaldehyde and acetone | 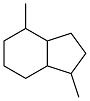 | 0.91 | -44 | — | — | 40 |
| 4-ethylbenzaldehyde and acetone | 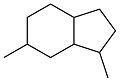 | 0.94 | -41 | — | — | 40 |
| Cyclopentanone and vanillin | 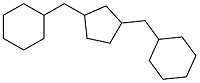 | 0.943 | -35 | — | — | 27 |
| Cyclopentanone and vanillin | 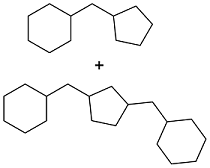 | 0.89 | < -60 | — | — | 28 |
| 2-methylfuran and dicyclopentadiene | 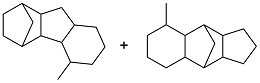 | 0.984 | -58 | 41.96 | 15.5 (20 ℃) | 45 |
| β-Pinenes | 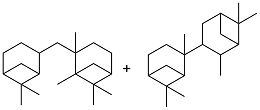 | 0.94 | < -30 | 39.5 | 4199 (-10 ℃) | 32 |
| Cyclohexanone and vanillin | 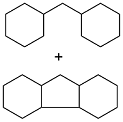 | 0.95 | -17 | 39.3 | — | 43 |
| 2-methyl-2,4-pentanediol and p-quinone | 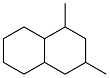 | 0.91 | -48∽-27 | — | — | 53 |
| Isophene and p-quinone | 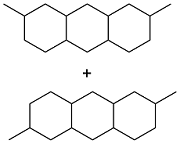 | — | — | 45.7 MJ/kg | — | 54 |
| Cyclohexanol and methylcyclopentane | 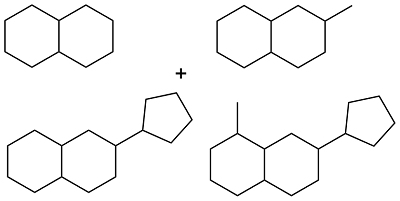 | 0.90 | < -72 | 38.0 | 4.3 (20 ℃) | 62 |
| Phenol and cyclopentanol | 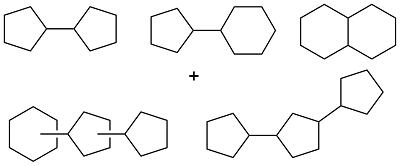 | 0.88 | < -75 | 37.4 | 3.5 (20 ℃) 10.4 (-20 ℃) | 36 |
| Cyclopentanone and cyclohexanone | 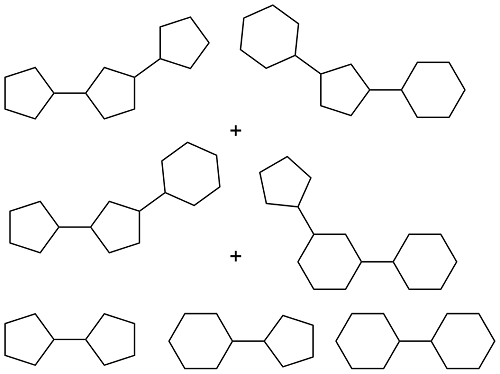 | 0.905 | < -50 | 38.67 | 7.6 (20 ℃) | 26 |
| Lignin oil and cyclopentanol | 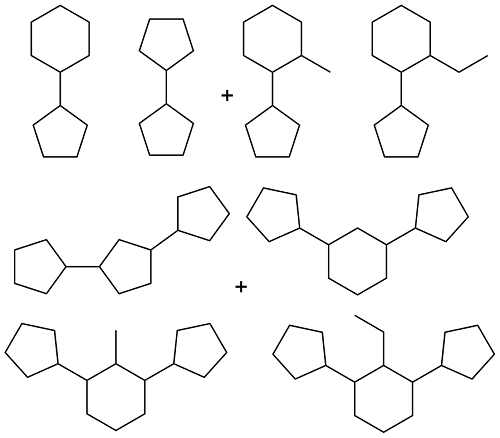 | 0.91 | < -60 | 39.0 | 5.59 (20 ℃) | 37 |
| [1] |
(邹吉军, 张香文, 王笠, 米镇涛. 含能材料, 2007, 15(4): 411.)
|
| [2] |
(邹吉军, 郭成, 张香文, 王笠, 米镇涛. 推进技术, 2014, 35(10): 1419.)
|
| [3] |
|
| [4] |
|
| [5] |
(潘伦, 邓强, 鄂秀天凤, 聂根阔, 张香文, 邹吉军. 化学进展, 2015, 27(11): 1531.)
|
| [6] |
(谢嘉维, 张香文, 谢君健, 聂根阔, 潘伦, 邹吉军. 化学进展, 2018, 30(9): 1424.)
|
| [7] |
|
| [8] |
|
| [9] |
(王贞, 卫豪, 贺芳, 卫宏远. 导弹与航天运载技术, 2011, 3: 41.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
(朱本强, 袁冰, 于凤丽, 解从霞. 青岛科技大学学报(自然科学版), 2022, 43(5): 14.)
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
/
| 〈 |
|
〉 |