

Spatial Omics and Clinical Imaging Technique for Accurate Diagnosis of Tumor
Received date: 2024-01-15
Revised date: 2024-02-01
Online published: 2024-02-07
Supported by
National Natural Science Foundation of China(22176195)
Guangdong Province Zhu Jiang Talents Plan(2021QN02Y028)
Key Program of Fundamental Research in Shenzhen(JCYJ20210324115811031)
Shenzhen Key Laboratory of Precision Diagnosis and Treatment of Depression(ZDSYS20220606100606014)
Clinical Research Project of Shenzhen Second People’s Hospital(2023yjlcyj003)
Scientific research fund of Shenzhen Health Economics Association(2023107)
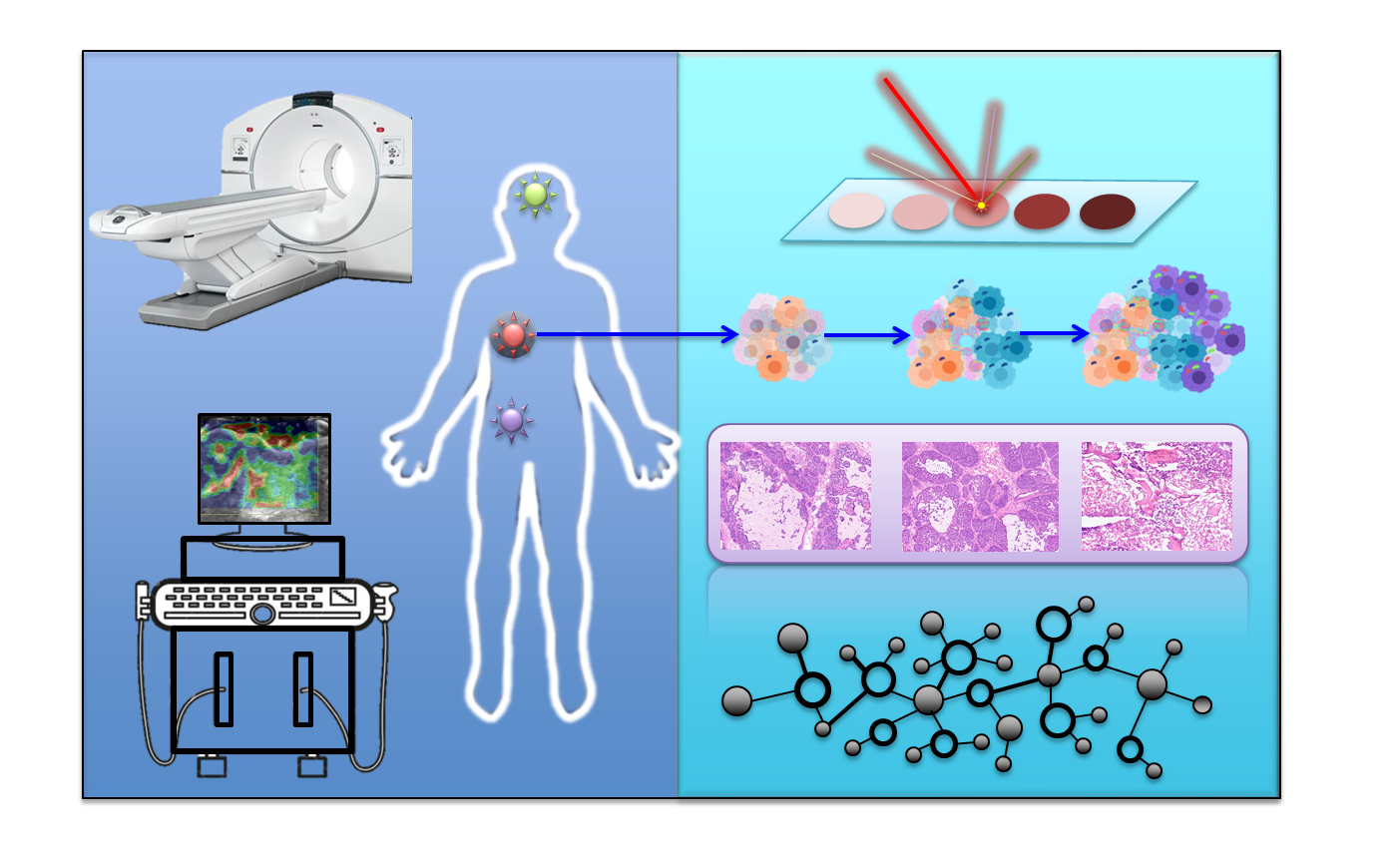
As a global public health event, the emergence of malignant tumors seriously affects human health, longevity and quality of life. The occurrence and development of tumors undergo extremely complex processes, showing high spatial and temporal heterogeneity in multiple bio-information and disease progression. These features affect tumor metastasis and drug resistance. In order to explore tumor heterogeneity, a variety of clinical imaging techniques and spatial omics techniques have been rapidly developed. Tumor characteristics are accurately evaluated by using the conventional clinical imaging technique with a non-invasive advantage. However, it is difficult to obtain accurate tumor staging and more molecular information using clinical imaging techniques. Spatial omics technology can be used to determine a variety of cell types, spatial and temporal distribution, molecular typing, and molecular interaction networks, thereby obtaining the accurate panoramic spectrum in tumor biology. Although spatial omics technique can detect a variety of molecules and their interactions, such as genes, proteins, and metabolites, as well as the interactions between gene-gene, protein-protein, metabolite-metabolite, gene-protein, gene-metabolite, and protein-metabolite, it can’t provide in vivo information. The combination of clinical imaging and spatial omics technology can complement advantages and has great application prospects in clinical and basic scientific research. The novel fusion technique plays an important role in promoting the accurate analysis of the spatio-temporal heterogeneity of tumors, the identification of molecular typing, and the accurate diagnosis and prediction of tumor progression. Herein, we summarize the strategies and characteristics of this novel fusion technique in the accurate diagnose of tumors and also prospect for future development.
Contents
1 Introduction
2 Spatial omics
3 Clinical imaging
4 Spatial omics and clinical imaging fusion
5 Perspectives
Peng Zhou , Zongwei Cai , Chao Zhao . Spatial Omics and Clinical Imaging Technique for Accurate Diagnosis of Tumor[J]. Progress in Chemistry, 2024 , 36(2) : 159 -166 . DOI: 10.7536/PC240113
图1 人体乳腺癌标本的空间代谢组-空间蛋白组联合分析:组织学分析(a)、空间分割(b)和多元统计分析(c~e)揭示了肿瘤脂质代谢分布的异质性;肿瘤组织脂质空间成像(f)和蛋白质空间成像(g)[24]Fig.1 Spatial metabolomics and proteomics analysis of human breast cancer sample. (a) Histological analysis, (b) spatial segmentation and (c~e) multivariate statistical analysis revealed the intratumor heterogeneity of lipid distribution; (f) lipid imaging and (g) protein imaging from the on-tissue digestion method[24]. Copyright 2021 Elsevier |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
Spatial Omics DataBase (SODB). Nat. Meth., 2023, 20(3): 359.
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
/
| 〈 |
|
〉 |