

In-situ Preparation Methods of Hydrogen Peroxide via Water Oxdation
Received date: 2023-06-19
Revised date: 2023-11-11
Online published: 2024-01-08
Supported by
National Natural Science Foundation of China(22172057)
Hydrogen peroxide (H2O2) is an important chemical that may be used as a clean disinfectant. For scale application, H2O2 is produced primarily by the anthraquinone process. The necessary transportation and storage processes bring explosion risks, so it is urgent to develop in-situ preparation methods. Electrochemical and photocatalytic reduction of oxygen to product H2O2 have received wide attention, but these reactions are carried out at the gas-liquid-solid interface. This three-phase reaction requires complex equipment and sequentially limits large-scale production. Another equally important pathway for in-situ H2O2 production is the oxidation of water which needs only solid-liquid two-phase interface. This paper summarizes the common methods of oxidizing water to prepare H2O2, such as electrochemistry and photocatalysis, and focuses on the recent new methods of in-situ H2O2 preparation, including thermal catalysis, ultrasonic piezoelectricity, plasma and microdroplet method. These methods provide the references for in-situ H2O2 production and in particular its utilization in the field of disinfection.
Contents
1 Industrial process for the production of hydrogen peroxide
2 In-situ production of hydrogen peroxide via oxygen reduction reaction
3 In-situ production of hydrogen peroxide via water oxidation reaction
3.1 Electrochemical and photocatalytic hydrogen peroxide generation from water oxidation
3.2 Thermocatalytic hydrogen peroxide generation
3.3 Ultrasonic piezoelectrical hydrogen peroxide generation
3.4 Electrical discharge plasma hydrogen peroxide generation
3.5 Generation of hydrogen peroxide from aqueous microdroplets
4 Conclusion and outlook
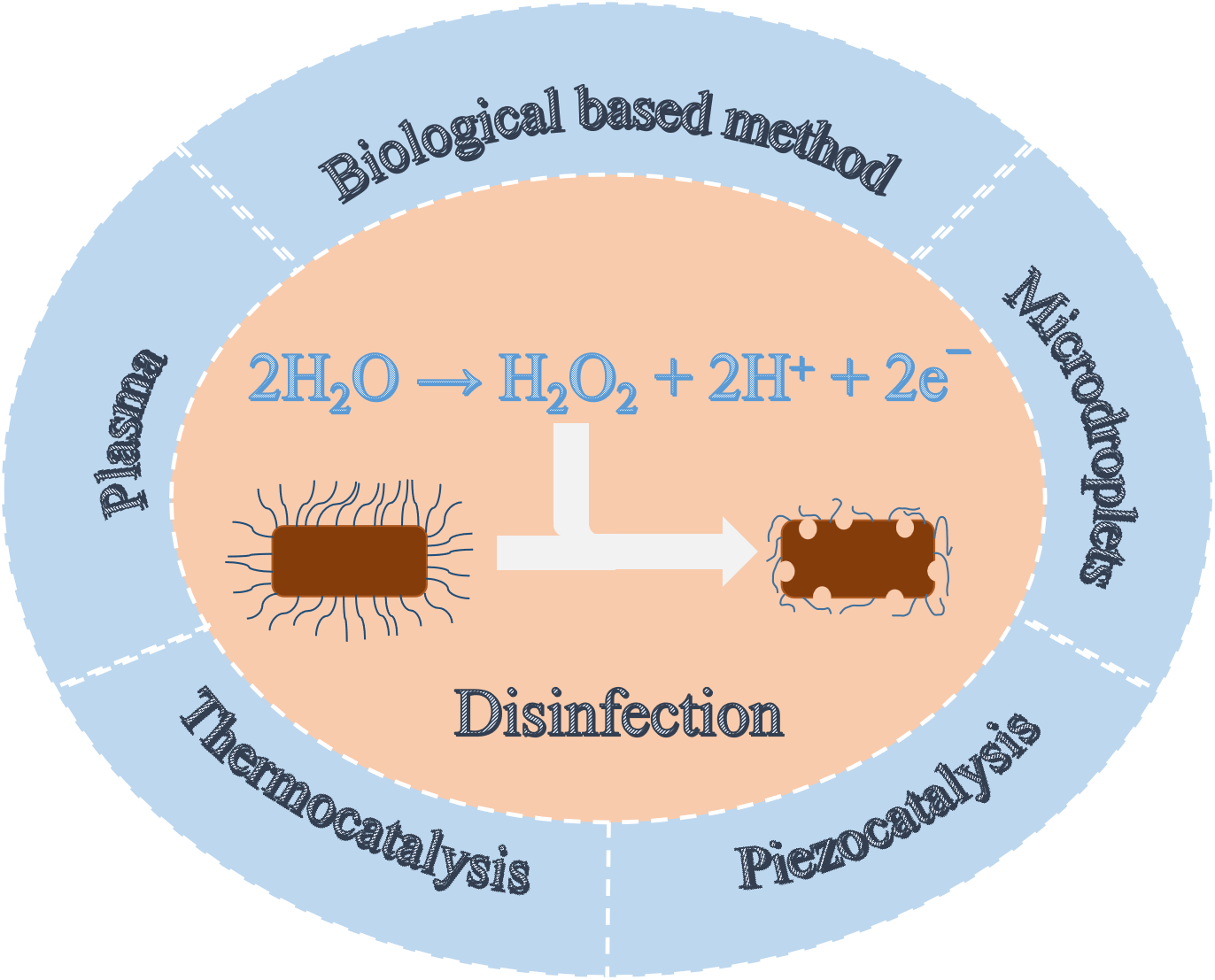
Key words: disinfection; hydrogen peroxide; in-situ preparation; water oxidation
Jingze Yu , Tengfeng Xie . In-situ Preparation Methods of Hydrogen Peroxide via Water Oxdation[J]. Progress in Chemistry, 2024 , 36(2) : 177 -186 . DOI: 10.7536/PC230613
表1 以水为原料现场产H2O2方法的对比Table 1 Performance comparison of in-situ H2O2 preparation via water oxdation |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
孙世雄, 樊晓旭, 孙明军, 张皓博, 田莉莉, 张培培, 孙淑芳. 中国消毒学杂志, 2022, 39(7): 494.)
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
杨盟飞, 冯彬, 白立光, 赵晓东. 化学推进剂与高分子材料, 2024, 22(1): 25.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
(刘嚆, 薛飞飞. 中华纸业, 2022, 43(6): 24.)
|
| [14] |
(梁海瑞, 王涖, 刘国柱. 化工进展, 2021, 40(4): 2060.)
|
| [15] |
|
| [16] |
|
| [17] |
(沈培康. 电化学氧还原的理论基础和应用技术. 南宁: 广西科学技术出版社, 2018. 11.)
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
奚文灏, 兰彦, 沈洁, 韩伟, 程诚. 安徽大学学报(自然科学版), 2022, 46(3): 3.)
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
/
| 〈 |
|
〉 |