

Synthesis and Application of Ion-Doped Mesoporous Bioactive Glasses
Received date: 2023-06-10
Revised date: 2023-10-25
Online published: 2024-01-08
Supported by
Science and Technology Research Project of Henan Province(222102310112)
Science and Technology Research Project of Henan Province(222102320028)
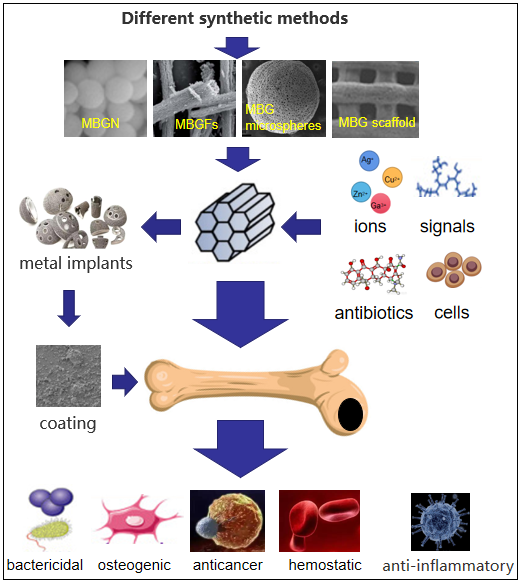
The research and development of bone filling and bone substitute biomaterials is one of the important research directions in the field of bone repair. Mesoporous bioactive glass (MBG) will play an important role in bone repair and regeneration because of its good bioactivity, adjustable pore size and ordered mesoporous structure. MBG with fiber, scaffold, hollow structure or nano-particle structure can be obtained by different preparation and processing methods. Many studies have shown that the incorporation of a small amount of therapeutic inorganic ions into MBG can endow them with more biological properties, including osteogenic, antibacterial, anti-inflammatory, hemostatic or anti-cancer properties. Moreover, MBG doped with inorganic ions still has excellent bioactivity after being processed as scaffolds or nanoparticles. In addition, the performance of MBG can be further improved by loading bioactive molecules, therapeutic drugs and stem cells into the mesoporous structure. In this paper, the synthesis of MBG, the antibacterial properties of metal ion-doped MBG and the application of MBG in other fields are reviewed.
Contents
1 Introduction
2 MBG synthesis
2.1 Preparation of MBG nanoparticles
2.2 Preparation of MBG fiber (MBGF)
2.3 Preparation of MBG microspheres
2.4 Preparation of MBG scaffold
3 MBG used as antimicrobial carrier
3.1 MBG as an antibiotic carrier
3.2 MBG used as antibacterial ion carrier
3.3 MBG doped with other antiseptic
4 MBG in other applications
4.1 MBG used in hemostasis
4.2 MBG used in anti-inflammation
4.3 MBG used in anti-cancer
4.4 MBG as coating material
5 Conclusion and prospect
Qiwei Li , Jianguo Liao . Synthesis and Application of Ion-Doped Mesoporous Bioactive Glasses[J]. Progress in Chemistry, 2024 , 36(2) : 271 -284 . DOI: 10.7536/PC230610
图2 (a)Ag@pMBG 制备的示意图,即通过邻苯二酚基团的氧化还原活性在 MBG 介孔通道的内表面和外表面原位还原 Ag+ 至 Ag 纳米颗粒。(b)用选择性激光烧结制备多孔复合支架的示意图。典型多孔支架(PLLA-PGA/ Ag@pMBG 和 PLLA-PGA/MBG)的宏观形貌(光学图像)表现出均匀的孔和支架结构[83]Fig. 2 (a) Schematic of Ag@pMBG preparation, in situ reduction of Ag+ to Ag nanoparticles on the inner and outer surfaces of mesoporous channel of MBG by redox activity of catechol groups. (b) Schematic for preparation of porous composite scaffolds by selective laser sintering. The macroscopic morphology (optical images) of the typical porous scaffolds (PLLA-PGA/Ag@pMBG and PLLA-PGA/MBG) demonstrated a uniform pore and strut structure[83] |
表1 离子掺杂MBG抗菌的主要研究汇总Table 1 Summary of major studies on ion-doped MBG antibacterial agents |
| Ion(s) doped | Composition | Synthesis method | Biocompatibility | Bacterial species | Antibacterial properties | Refs |
|---|---|---|---|---|---|---|
| Ag+/ Ag | PLLA-PGA/xAg@pMBG (x=0/2.0/4.0/6.0/ 8.0mol%) | Sol-Gel | The cells cultured on the scaffold with MBG exhibited a flatter morphology, indicating better cytocompatibility. the degradation of MBG releases active elements (silicon and calcium) that could induce osteoblast differentiation. | E. coli | PLLA-PGA/MBG scaffolds had no antibacterial activity; The bacteriostasis rates of composite scaffolds loaded with 2AG@PMBG and AG@PMBG were 80.0% and 83.0% ,respectively; The bacteriostasis rate of composite scaffolds loaded with 6AG@PMBG and 8Ag@pMBG was more than 99.00%; | 83 |
| Zn2+ | 70SiO2-(30-x)CaO-xZnO (x=0/2.0/4.0mol%) | Sol-Gel | All samples show very high levels of cell mitochondrial activity (> 80.0% of the reference control). The results indicate that all glasses are not cytotoxic (viability is always above 80.0%, indicates that the material should be considered as not cytotoxic) and favorable for cell proliferation. | E. coli S.aureus | When the ratio of the extract to the bacterial mixture was 1ml: 0.5 ml, the bacteriostasis rates of 2Zn-MBG to both bacteria were 100.0%, and the bacteriostasis rates of 4Zn-MBG to E. coli and S. aureus were 65.0% and 70.0%, respectively; When the ratio was 1ml: 0.2 ml, the inhibition rates of 2Zn-MBG to both bacteria were 100%, while those of 4Zn-MBG to E. coli and S. aureus were 80.0% and 85.0%,respectively; | 92 |
| Cu2+ | 45SiO2-6P2O5-24.5CaO-(24.5-x)Na2O (x=0/0.5/1.5/2.5 mol%) | Sol-Gel | Ionic radii variations can influence the dissolution of calcium and phosphate, so apatite growth was gradually increased by addition of copper in BG system. BG and copper incorporated BG showed almost similar bioactivity as well as exhibiting same apatite growth with the additional benefit of copper release. | P.aeruginosa E. coli B.subtilis S. aureus E.faecalis C.albicans | Under the treatment of 1.5 cu-mbg and 2.5 cu-mbg, the viable cells of Pseudomonas aeruginosa and E. coli were completely inhibited, and the inhibitory effects on B. subtilis and S. aureus were obviously superior to those of Gram-negative; CuBGs (20.0 μg/ml) had a rapid inhibitory effect on Gram-positive bacteria such as Enterobacter faecalis, Candida albicans and Staphylococcus aureus, with inhibition rates of 98.5%, 99.0% and 98.5%, respectively; | 97 |
| Ce3+ | 88.5SiO2-10.1 CaO-1.4Ce2O3 | Sol-Gel | some apatite particles existed as hollow hemispheres on day 3 and day 7. as the immersion time increased to 14 days, when most of the apatite particles had grown into full spheres. All samples induced the formation of apatite particles with Ca/P ratio close to 1.67 upon immersion in simulated body fluid (SBF), confirming their good bioactivity. | E. coli S. aureus | Ce-MBG has antibacterial activity against Gram-positive bacteria and Gram-negative bacteria by producing reactive oxygen species; When the concentration was 0.01 mg/ml, it had no effect on E. coli, but the survival rate decreased with the increase of MBG concentration; When the concentration was 10.0 mg/ml, the growth of Staphylococcus aureus was inhibited completely; | 113 |
| Ce3+/ Ga3+ | 60.0SiO2-(40.0- x)CaO-xGa2O3 (x=0/1.0/3.0/5.0mol%) | Sol-Gel | The Ca/P ratio on the MBGNPs surface was close to 1.64, which is similar to the Ca/P ratio in HA. All Ga-doped MBGNPs showed the formation of a similar type of HA crystals on the surface. Increasing the amount of gallium doping resulted in significant refinement of precipitated HA crystals. | E. coli S. aureus | Ga-MBG had a lower survival rate than Gram-positive bacteria and Gram-negative; The inhibition rate of 5Ga-MBG was the highest at 6h; Ga1 was the strongest at 6h and 24h after Gram-positive bacteria Gram-negative; The inhibition rates of Ga1, Ga3 and Ga5 MBG on Gram-negative were significantly different at 24 h incubation. | 120 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
Ladrón de Guevara-Fern S. Biomaterials, 2003, 24(22): 4037.
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [39] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
/
| 〈 |
|
〉 |