

Application of MOFs-Derived Metal Sulfides and Their Composites in Photocatalysis
Received date: 2023-08-15
Revised date: 2024-01-13
Online published: 2024-03-15
Supported by
Guangdong Science and Technology Innovation Strategy Program(2023S005054)
With the rapid development of society and economy,the demand for energy has been continuously increasing.Solar energy has emerged as a clean energy source with great development potential,and the long-term effective utilization of solar energy has become an urgent problem that needs to be addressed.Metal-organic frameworks(MOFs)derived metal sulfides retain the original structural characteristics of their parent MOFs,including large surface areas,dispersed nanoscale subunits,and abundant active sites.They overcome the limitations of MOFs in terms of material stability at high temperatures and harsh chemical environments.Moreover,compared to metal oxides,they have a narrower bandgap,which extends their light absorption range to the visible region.The porous nature of MOFs-derived metal sulfides also provides additional pathways for light-induced electron migration,promoting charge carrier separation.As a result,they have attracted increasing attention in the field of photocatalysis.Although this field is still in its nascent stage,the results obtained so far indicate that MOF-derived metal sulfides and their composite photocatalysts have high potential for practical applications.This article systematically elucidates the synthesis,performance,and mechanisms of MOFs-derived metal sulfide photocatalysts and their composites in various application areas,such as wastewater treatment,water splitting for hydrogen generation,and CO2reduction.This will provide a new direction for the synthesis and application of novel and efficient composite photocatalytic materials.Additionally,some existing issues in current research are addressed,and the future prospects and challenges of MOFs-derived sulfide photocatalytic materials are discussed 。
1 Introduction
2 Strategies for the preparation of MOFs-derived metal sulfides
3 MOFs-derived monometallic sulfides
4 MOFs-derived polymetallic sulfides
5 MOFs-derived multicomponent metal sulfides
6 MOFs-derived oxide-sulfide composites
7 MOFs-derived carbon-based-sulfide,carbon-nitrogen-based-sulfide composites
8 Conclusion and outlook
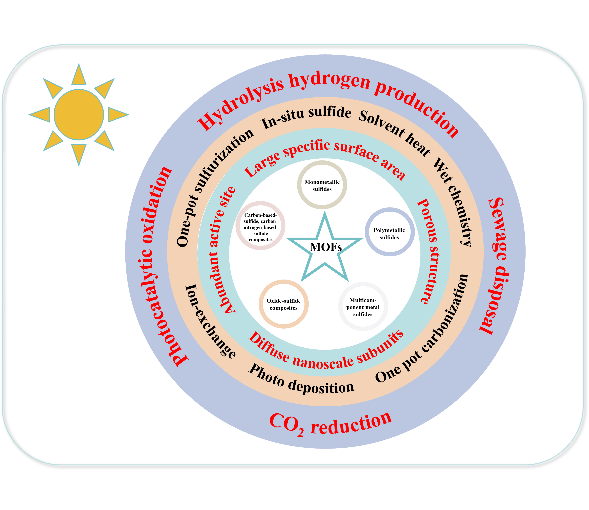
Key words: metal-organic frameworks(MOFs); metal sulfides; photocatalytic; synthesis
Chen Shijian , Pan Yuxuan , Cheng Lihua , Qian Junfeng , Wang Hui . Application of MOFs-Derived Metal Sulfides and Their Composites in Photocatalysis[J]. Progress in Chemistry, 2024 , 36(4) : 511 -524 . DOI: 10.7536/PC230806
表1 Application of MOFs-derived metal sulfides and their composites in photocatalysisTable 1 Application of MOFs-derived metal sulfides and their composites in photocatalysis |
| MOFs | Sample | Applications | Performance | ref |
|---|---|---|---|---|
| ZIF-L | ZnS | Cr(VI) reduction; X-3B degradation | 100% (30 min, UV Light); 94.6% (20 min, UV Light) | 17 |
| MIL-68-In | In2S3 | MO degradation | 97% (120 min, >420 nm) | 18 |
| Zn-MOF; Cd-MOF | ZnS; CdS | MB degradation | 90.2%; 91.6% (100 min, >420 nm) | 19 |
| Sn-MOF | SnS2-SnS | Cr(Ⅵ) reduction | 91.51% (180 min, 400~800 nm) | 20 |
| CdIF-3 | BW@CdS/Cd2SO4(OH)2 | H2 evolution | 18420 μmol·h-1·g-1 (Pt, Lactic acid, >420 nm) | 21 |
| ZIF-8 | Cd0.42Zn0.58S | H2 evolution | 4100 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 22 |
| ZIF-8 | Cd0.5Zn0.5S | Cr(Ⅵ) reduction; MO degradation | 100% (12 min, >420 nm); 94.2% (20 min, >420 nm) | 23 |
| ZIF-67 | Cu0.9Co2.1S4@MoS2 | H2 evolution | 40156 μmol·h-1·g-1 (TEOA, >420 nm) | 24 |
| ZIF-8 | Zn0.7Cd0.3S/CdS | H2 evolution | 2700 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 25 |
| Cd-MOF | (Zn0.95Cu0.05)0.6Cd0.4S | H2 evolution | 4150.1 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 26 |
| ZIF-67 | Co3S4@Mo2S3 | H2 evolution | 75 μmol·h-1·g-1 (TEOA, 420 nm) | 27 |
| Ni-ZIF-67 | NiS/Co3S4/ZnCdS | H2 evolution | 98600 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 28 |
| Ni-MOF | NiS/CdS | H2 evolution | 57930 μmol·h-1·g-1 (Lactic acid, 420 nm) | 29 |
| Cd-MOF | NiS/Zn0.5Cd0.5S | H2 evolution | 16780 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 30 |
| ZIF-8 | CIZS | H2 evolution; Photo-reforming reaction | 74.8 μmol·h-1·g-1 (360 nm) AQY of α-cellulose: 28% | 31 |
| ZIF-8 | ZnS/ZnIn2S4 | H2 evolution | 453.4 μmol·h-1·g-1 (TEOA, Xenon Light) | 32 |
| Ni-MOF-74 | Cd-Ni-MOF-T/CdS/NiS | H2 evolution | 40080 μmol·h-1·g-1 (Lactic acid, >420 nm) | 33 |
| MIL-68(In) | MnS/In2S3 | CO2 reduction | 58 μmol·h-1·g-1 (TEOA, Xenon Light) | 34 |
| ZIF-67 | Co9S8-Mn0.05Cd0.95S | H2 evolution | 13369 μmol·h-1·g-1 (Na2S-Na2SO3, UV Light) | 35 |
| ZIF-67 | ZnIn2S4@Co3S4 | H2 evolution | 4261 μmol·h-1·g-1 (TEOA, ≥350 nm) | 36 |
| ZIF-67 | ZnIn2S4@CoS2 | H2 evolution | 2768 μmol·h-1·g-1 (TEOA, >350 nm) | 37 |
| MOF-74-Ni/Cd | CdS@NiS | H2 evolution | 42700 μmol·h-1·g-1 (Lactic acid, 450 nm) | 38 |
| ZIF-8 | ZnS@SnS2/CdS | CO2 reduction | 155.57 μmol·h-1·g-1 (300 ~ 1100 nm) | 39 |
| Cd-MOF | CdS/MoS2 | H2 evolution | 5587 μmol·h-1·g-1 (Lactic acid, >420 nm) | 40 |
| Co-MOF | Co4S3/CdS | H2 evolution | 5892.6 μmol·h-1·g-1 (Lactic acid, 420 nm) | 41 |
| ZIF-8 | Sv-ZnS/ZnIn2S4 | H2 evolution; CO2 reduction | 2912.3 μmol·h-1·g-1; 2075.7 μmol·h-1·g-1 (TEOA, >420 nm) | 42 |
| Mo-MOF; Cd-MOF | MoS2/CdS | H2 evolution | 106729 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 43 |
| ZIF-67 | Co9S8/CdS | H2 evolution | 1852 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 44 |
| Co-MOF | Co4S3/CdS | H2 evolution | 12360 μmol·h-1·g-1 (Lactic acid, >420 nm) | 45 |
| ZIF-8 | Cu/ZnS-TAA | MB degradation; MO degradation | 260 mg/g (10 min, >420 nm) | 46 |
| CuZn-MOF | CuS/ZnS | H2 evolution | 6208.6 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 47 |
| ZIF-8 | CeO2/ZnS-CuS | H2 evolution | 13470 μmol·h-1·g-1 (Methanol, Xenon Light) | 48 |
| ZIF-67 | TiO2-Ti3C2-CoSx | H2 evolution | 950 μmol·h-1·g-1 (Methanol, UV-visible light) | 49 |
| ZnCo-ZIF@ZIF-8 | Co3O4-ZnO@ZnS/Pt | H2 evolution | 3269.3 μmol·h-1·g-1(Na2S-Na2SO3, Xenon Light) | 50 |
| MOF-5 | ZnO/ZnS | H2 evolution | 415 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 51 |
| Mo-MOF | NPC-MoS2@Bi4O5Br2 | CO2 reduction | 19.16 μmol·h-1·g-1 (380 nm) | 52 |
| Cd-MOF | CdS/NC-500 | TC degradation | 83% (60 min, >420 nm) | 56 |
| Mo-MOF | MoS2/CN | H2 evolution | 423.94 μmol·h-1·g-1 (TEOA, >420 nm) | 57 |
| ZIF-67 | CoSx-C/N2@CZS | H2 evolution | 18570 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 58 |
| ZIF-8 | g-C3N4/ZnS | MO degradation | 74.40% (160 min, >420 nm) | 59 |
| CuZn-ZIF@ MIL-68(In) | Cu0.5Zn0.5In2S4-rGO -g-C3N4 | H2 evolution | 11600 μmol·h-1·g-1 (Na2S-Na2SO3, >420 nm) | 60 |
| In-MOF | In6S7 NPs | MO degradation | 88% (160 min, 400~700 nm) | 61 |
| Ni-MOF | g-C3N4/C@Ni3S4/Ni2P | H2 evolution | 14490 μmol·h-1·g-1 (>420 nm) | 62 |
| Cu-Ni-MOF | Cu2S:NiS2@C/rGO | Photocatalytic C-N coupling application of phenylboronic acid and imidazole | Conversion rate and selectivity > 90% | 63 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
( 代振. 中原工学院硕士论文, 2022.)
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
( 徐建华, 谈玲华, 寇波, 杭祖圣, 姜炜, 郏永强. 化学进展, 2016, 28 (01): 131.)
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
/
| 〈 |
|
〉 |