

Coupling Different Synthesis Routes to Prepare Zeolite Molecular Sieves
Received date: 2023-08-15
Revised date: 2023-11-27
Online published: 2024-02-26
Supported by
National Natural Science Foundation of China(22168022)
Basic Research Innovation Group Project of Gansu Province(22JR5RA219)
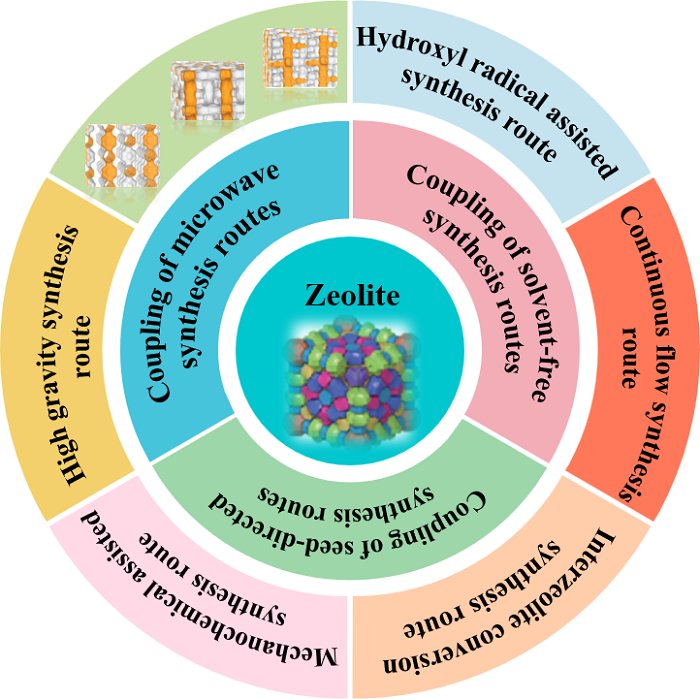
zeolites as one kind of important porous materials have a wide range of applications in the fields of adsorption,ion exchange,and catalysis due To their special pore structure,suitable acidity,and high hydrothermal stability.the classical hydrothermal route of synthesizing zeolites suffers from low zeolite yields,high synthesis costs,and serious pollution.coupling the classical synthesis routes of zeolites with those recently developed new non-conventional routes is an important way to solve the above problems and achieve technological innovation in zeolite synthesis.to this end,this review firstly introduces the principles and characteristics of those non-conventional synthesis routes of zeolites,then briefly introduces the concepts of coupling synthesis routes of zeolites,and then focuses on the latest progress in the preparation of zeolites by microwave synthesis,solvent-free synthesis,and seed-directed synthesis coupled with other routes.Finally,the main problems existing in these coupling routes are analyzed and viewed。
1 Introduction
2 Introduction to the non-conventional synthesis routes of zeolites
2.1 Hydroxyl radical assisted synthesis route
2.2 Continuous flow synthesis route
2.3 Interzeolite conversion synthesis route
2.4 Ultragravity synthesis route
2.5 Mechanochemical assisted synthesis route
3 Coupling of microwave synthesis with other synthesis routes
3.1 Microwave-hydrothermal synthesis route
3.2 Microwave-ionothermal synthesis route
3.3 Microwave-solvent-free synthesis route
4 Coupling of solvent-free synthesis with other synthesis routes
4.1 Solvent-free and hydroxyl radical assisted synthesis route
4.2 Solvent-free and seed directed synthesis route
4.3 Solvent-free and mechanochemical assisted synthesis route
5 Coupling of the seed directed synthesis with other synthesis routes
5.1 Seed directed and hydroxyl radical assisted synthesis route
5.2 Seed directed and continuous flow synthesis route
6 Other coupling routes
6.1 Hydrothermal-seed-ultragravity synthesis route
6.2 Ionothermal-interzeolite conversion synthesis route
7 Comparison of various coupling synthesis routes of zeolites
8 Conclusions and outlook
Zhao Xinhong , Wang Hao , Ding Mengqi , Li Hongwei , Ji Dong , Li Guixian . Coupling Different Synthesis Routes to Prepare Zeolite Molecular Sieves[J]. Progress in Chemistry, 2024 , 36(4) : 525 -536 . DOI: 10.7536/PC230802
表1 Characteristics of Zeolite Synthesis by Coupling Microwave Synthesis with Other Synthetic RoutesTable 1 Characteristics of microwave synthesis coupled with other routes in preparing zeolites |
| Microwave coupling routes | Advantage | Disadvantage | Framework | Ref |
|---|---|---|---|---|
| Microwave-hydrothermal synthesis route | Owing to the advantages of rapid and uniform heating of microwave compared to conventional heating, the crystallization process of zeolites with microwave heating is more rapid to enter the crystal growth period, and the crystallization time is evidently shortened, making the process more energy saving. | The experimental process of microwave hydrothermal routes is sometimes not safe, and organic templating agents or volatile solvents can still cause problems such as excessive pressure generation. | MFI, CHA, FAU, LTA, AFI, *BEA | 10⇓⇓⇓~14 |
| Microwave-ionothermal synthesis route | The ionic liquid is a good microwave absorber. The combination of microwave heating and ionic liquid can make heating more uniform, provide a faster heating rate, and allow for a shorter aging time. | The number of ionic liquids is limited in variety, the reaction mechanism of the microwave-ionothermal synthesis route still lacks understanding, and its industrial application requires further research. | AEL, AST, Fe-LEV, AFI | 15⇓⇓⇓⇓⇓⇓~22 |
| Microwave-solvent-free synthesis route | Microwave and solvent-free coupling has the advantages of being fast and safe, having low energy consumption, andreducing the generation of wastewater. | Not any of the synthesis reactions can occur in a microwave solvent-free system, and the types of molecular sieve synthesis are limited. | AFI | 23 |
表2 Characteristics of Zeolite Molecular Sieve Preparation by Coupling Solvent-free Synthesis Route with Other TechnologiesTable 2 Characteristics of solvent-free synthesis coupled with other techniques in preparing zeolites |
| Solvent-free coupling routes | Advantage | Disadvantage | Framework | ref |
|---|---|---|---|---|
| Solvent-free hydroxyl radical synthesis route | The introduction of hydroxyl radicals can appropriately reduce the amount of alkali in the synthesis system as well as the amount of organic templating agent and reduce the energy consumption in the synthesis process by improving the zeolite crystallization rate. | Only a small number of zeolite molecular sieves can be synthesized via this route, and the promotion mechanism of hydroxyl radicals in the solvent-free condition still lacks understanding. | Fe-MFI | 28 |
| Seed-directed solvent-free route | The raw materials can be fully mixed with seeds by grinding under solvent-free conditions, which is conducive to the effective collision and spontaneous diffusion of molecules and helps all the reactants to participate in the synthesis reaction. The addition of an appropriate amount of seeds can shorten the induction period and inhibit the generation of other hetero-crystalline phases, which significantly reduces the synthesis time. | The reaction is usually carried out in an immobile solid-solid system, resulting in the poor efficiency of heat conduction, which severely limits large-scale industrial applications. | *BEA, MFI, CHA | 32⇓⇓~35 |
| Solvent-free mechanochemical-assisted route | The coupling of solvent-free and mechanochemical methods makes up for the shortcomings of manual grinding, which is prone to producing amorphous products, and the high-energy ball milling process makes the mixing of the initial raw materials more homogeneous, which helps to increase the reactivity, and the operation process is simple and low-cost. | Ball milling media and rapid powder movement processes are difficult to monitor, and most studies have been conducted in planetary ball mills for material pre-preparation, which is not yet available for large-scale processing. | CHA, ANA, CAN, GIS, SOD, FAU, LTA, MFI | 37⇓⇓~40 |
表3 Characteristics of Zeolite Synthesis by Coupling Seed-Directed Route with Other Synthetic RoutesTable 3 Characteristics of seed-directed synthesis coupled with other routes in zeolite preparation |
| Seed-directed coupling routes | Advantage | Disadvantage | Framework | ref |
|---|---|---|---|---|
| Seed-directed template-free synthesis route | On the one hand, the addition of seeds eliminates the use of organic templating agents, maximizing the savings in production costs; on the other hand, seeds can be used as templates to induce the formation of zeolite frameworks. | The synthesis process is cumbersome, and the types of molecular sieves that are synthesized are limited. | EON, *BEA, MFI, TON, MOR, BOG | 44⇓⇓⇓⇓⇓⇓~51 |
| Seed-directed interzeolite transformation synthesis route | The introduction of seeds with common composite building units with the target zeolite not only induces the generation of the target zeolite, but also accelerates the dissolution of the starting zeolite, accelerates the nucleation rate of the crystal, and shortens the crystallization time to a certain extent. | The reaction is usually carried out under hydrothermal conditions, with a large amount of waste liquid discharge and high reaction pressure, which poses certain safety risks. | *BEA, MFI, CHA,,STF, MTW | 54⇓⇓~57 |
| Seed-directed hydroxyl radical synthesis route | An effective combination of seeds with highly active hydroxyl radicals can not only shorten the induction period and improve the utilization of raw materials, but also induce product-directed synthesis. | The role of •OH and seeds in zeolite crystallization is not well understood at the molecular level, and problems such as the precise quantification of •OH have not yet been solved. | LTA, MFI | 58~59 |
| Seed-directed continuous flow synthesis route | A continuous flow reactor has a good heat and mass transfer effect, and the induction of seeds can strengthen the above effect further, making up for the thermal delay effect of the high-pressure reactor and reducing the synthesis time. This route not only improves the crystallization rate, but also effectively regulate the particle size of the product. | Reaction gel makes the system viscosity easy to cause clogging, making the utilization of raw materials slightly reduced. This coupling method is currently limited to the laboratory development stage, and has not yet achieved industrial production. | AFI, MFI | 61⇓⇓~64 |
表4 Characteristics of Zeolite Molecular Sieve Prepared by Other Coupling RoutesTable 4 Characteristics of other coupling routes of synthesizing zeolites |
| Other coupling routes | Advantage | Disadvantage | Framework | Ref |
|---|---|---|---|---|
| Hydrothermal-seed- ultragravity synthesis route | The ultragravity technology can improve the microscopic mixing efficiency, which in turn enhances the heat and mass transfer efficiency, facilitates the homogeneous dispersion of seeds in the initial gel, contributes to the homogeneous growth of the product, and shortens the crystallization time. | Basic theoretical research on the ultragravity technique is seriously lagging behind, and industrial scale-up research is difficult. | MFI、FAU | 65⇓⇓⇓⇓~70 |
| Ionothermal-interzeolite conversion synthesis route | The internalzeolite conversion route partly compensates for the defects of the long synthesis cycle of the ionothermal method. The addition of ionic liquid can reduce the use of organic template agents to a certain extent. The reaction can be carried out at atmospheric pressure, avoiding the safety hazards brought about by high pressure. | Most ionic liquids are relatively expensive, thus increasing the synthesis cost. The problem of recycling ionic liquids has not yet been solved, making large-scale production difficult. | MFI | 71 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
( 徐如人, 庞文琴, 于吉红, 霍启升, 陈接胜. 分子筛与多孔材料化学. 北京: 科学出版社, 2015. 219.)
|
| [6] |
( 王健羽, 张强, 闫文付, 于吉红. 高等学校化学学报, 2021, 42(1): 11.)
|
| [7] |
|
| [8] |
( 潘迪, 刘鹏, 张宏斌, 唐颐. 化学进展, 2020, 32(7): 873.)
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
( 张峻维, 段维婷, 赵新红. 精细化工, 2020, 37(3): 547.)
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
( 张培青, 刘思成, 郑淑琴. 无机化学学报, 2020, 36(2): 289.)
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
( 成尚元, 刘有智, 祁贵生. 化工进展, 2017, 36(2): 588.)
|
| [69] |
( 齐婷婷, 滕加伟, 史静, 初广文, 邹海魁, 罗勇, 张亮亮, 孙宝昌. 化工进展, 2021, 40(11): 6228.)
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
/
| 〈 |
|
〉 |