

Application of Carbon Dots and Its Composites in the Field of Photocatalytic CO2 Reduction
Received date: 2023-08-17
Revised date: 2024-01-15
Online published: 2024-03-15
Supported by
Natural Science Foundation of Shaanxi Province(2022JQ-132)
Innovation Capability Support Program of Shaanxi(2019-TD-021)
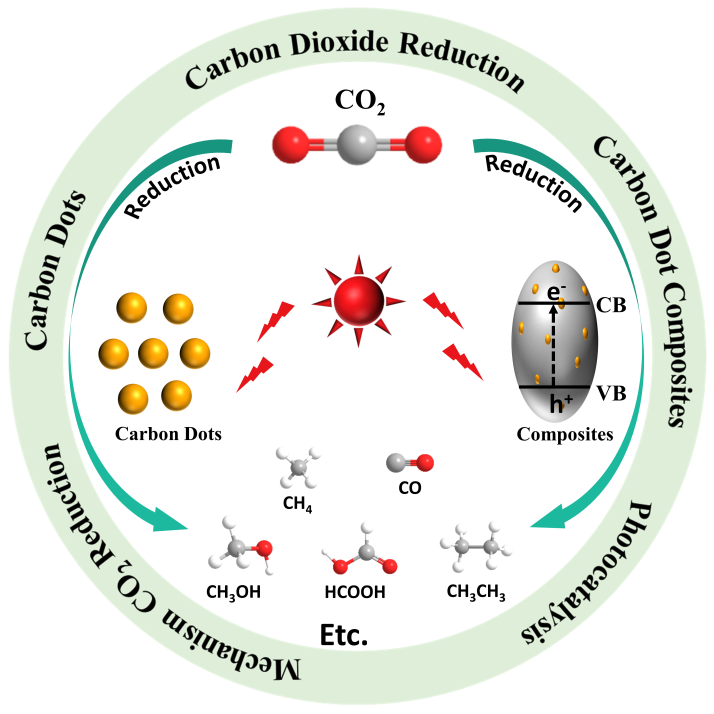
The massive consumption of fossil fuels has caused the continuous increase of carbon dioxide concentration in the atmosphere,resulting in serious climate and environmental problems such as greenhouse effect and sea level rise.The use of solar photocatalysis to reduce CO2to hydrocarbon fuel with added value is regarded as one of the most promising potential solutions.Researchers have developed a variety of photocatalysts,among which carbon dots are a new type of carbon nanomaterials with a size of less than 10 nm.They have unique up-conversion luminescence properties and can promote electron transfer.The synthesis method is friendly and safe.They are widely used in the field of photocatalytic reduction of CO2.In this paper,starting from the mechanism of photocatalytic reduction of CO2,the action mechanism and performance evaluation of carbon dots and carbon dot composite materials in photocatalytic reduction of CO2are reviewed in detail in terms of light absorption efficiency,carrier separation efficiency,CO2adsorption capacity and multiple interactions.The advantages of carbon dots in the field of photocatalytic reduction of CO2are summarized.The existing challenges and possible ways to address the challenges in the future are analyzed.And the future development is prospected.It provides a new idea for promoting the development of carbon dot-based photocatalysts 。
1 Introduction
2 Introduction of carbon dots
3 Mechanism of photocatalytic reduction of CO2by semiconductors
4 Application of carbon dots in photocatalytic reduction of CO2
4.1 Application of individual carbon dots in photocatalytic reduction of CO2
4.2 Application of Carbon Dots-Based Composite Materials in the Field of CO2Reduction
5 Conclusion and prospect
Dang Yongqiang , Huang Rui , Feng Xiangyu , Liu Guoyang , Zhu Youyu , Zhang Yating . Application of Carbon Dots and Its Composites in the Field of Photocatalytic CO2 Reduction[J]. Progress in Chemistry, 2024 , 36(4) : 575 -585 . DOI: 10.7536/PC230817
图1 (a) 由合适的氧化还原助催化剂介导的用于太阳能燃料生产的半导体光催化剂上光催化 CO2 转化的可能机理示意图[35];(b) 一些半导体光催化剂的能带位置和水溶液中 pH = 7 时 CO2 还原的氧化还原电位[36]Fig. 1 (a) Schematic illustration of probable mechanism of photocatalytic CO2 conversion over a semiconducting photocatalyst for solar fuels production mediated by suitable redox cocatalysts[35]; (b) Band positions of some semiconductor photocatalysts and the redox potentials of CO2 reduction at pH = 7 in aqueous solution[36] |
表1 Application of individual carbon dots in photocatalytic reduction of CO2Table 1 Application of individual carbon dots in photocatalytic reduction of CO2 |
| Catalyst | Synthesis method① | Product | Production rate | Selectivity | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| GCDs | Ultrasonic-assisted | CH4, CO | 983, 350 μmol·g-1·h-1 | 74.8% | CO2 adsorption | 46 |
| NH2-CNPs | reflux-stirring | CH3OH | 61.87 μmol·g-1·h-1 | 76.6% | CO2 adsorption site types and distribution | 47 |
| Fe-CDs | Hydrothermal | CH3OH | 654.28 μmol·g-1·h-1 | ─ | High electron transfer rate | 48 |
| TPP-CDs | Hydrothermal | CH3OH | 173.35 μmol·g-1·h-1 | ─ | sufficiently negative reduction potential, Light absorption | 49 |
①synthesis method of carbon dots |
图6 (a) CO2和CH3OH在CN上的吸附能和最稳定的构型,以及H2O在mCD上的吸附能;(b) mCD/CN和sCD/CN的光催化CO2还原示意图;(c)可见光下CN和mCD/CN上的CH3OH氧化试验[45]Fig. 6 (a) Adsorption energies and most stable configurations of CO2 and CH3OH on CN, and H2O on mCD; (b) Schematic illustration of the photocatalytic CO2 reduction of mCD/CN and sCD/CN; (c) CH3OH oxidation assay on CN and mCD/CN under visible light[45] |
表2 Application of carbon dots composite in photocatalytic reduction of CO2;Table 2 Application of carbon dot composites in photocatalytic reduction of CO2 |
| Catalyst | Synthesis methoda | Product | Production rate | Selectivity | The role of CDs | Ref. |
|---|---|---|---|---|---|---|
| BBQ | Thermal annealing | HCOOH | ─ | ─ | Charge separation | 11 |
| Au/CDs | Reflux | HCOOH, CH3COOH | ─ | ─ | Separate electron-hole pairs | 12 |
| Au-CDs | Irradiation | HCOOH | 4 μmol·g-1·h-1 | ─ | Separate electron-hole pairs | 13 |
| CPD/Bi4O5Br2 | Hydrothermal | CO and CH4 | 132.42 μmol·g-1·h-1 | ─ | Light absorption, Electron transfer | 19 |
| CQD-modified TiO2 NTs | Hydrothermal | CO and CH4 | 2.71, 0.71 μmol·g-1·h-1 | ─ | Light absorption, Electron transfer | 39 |
| CD@NH2-UiO-66 | Hydrothermal | CO | 16.6 μmol·g-1·h-1 | ─ | Charge transfer and separation | 42 |
| C/TiO2 | Calcination | CO | 0.86 μmol·g-1·h-1 | ─ | Electron transfer | 43 |
| mCD/CN | Microwave | CH3OH | 13.9 ± 1.7 μmol·g-1·h-1 | 99.6%±0.2% | Hole acceptor, Prevents the surface adsorption of methanol | 45 |
| Ni-PCD@TD-COF | Pyrolysis | CO | 396.5 μmol·g-1·h-1 | 98% | Light absorption | 50 |
| ZnO1-x /C | FuAR | CO | 60.77 μmol·g-1·h-1 | ─ | Light absorption | 52 |
| OD-ZnO/C | Calcination | CO | 118.8 μmol·g-1·h-1 | ─ | Light absorption | 53 |
| CQDs/UBW | Hydrothermal | CH4 | 0.899 μmol·g-1·h-1 | ─ | Light absorption, Electron transfer | 54 |
| NSCQDs/TiO2 | Ultrasound | CO and CH4 | 0.769, 1.153 μmol·g-1·h-1 | ─ | Light absorption, Electron transfer | 55 |
| CQDs/TiO2 | Calcination | CO | 46.21 μmol·g-1·h-1 | 100% | Electron transfer | 56 |
| CQDs/Cu2O | Stirring | C2H6 | 28.42μmol·g-1·h-1 | ─ | Electron transfer | 57 |
| CQDs/Cu2O | Calcination | CH3OH | 55.7 μmol·g-1·h-1 | ─ | Hole transfer | 58 |
| CL@CQDs/Cu2O | Hydrothermal | CH3OH | 99.6 μmol·g-1·h-1 | ─ | Light absorption, Electron transfer | 59 |
| CD/FAT | Suspend, Annealing | CH3OH | 24.2 μmol·g-1·h-1 | 100% | Hole acceptor | 60 |
| N-CQDs/Bi4MoO9 | Ultrasound | CO | 3.24 μmol·g-1·h-1 | ─ | Electron transfer, CO2 adsorption | 61 |
| Zn-Bim-His-1 @GQDs | Ultrasound, Stirring | CH4 and CO | 20.9, 3.7 μmol·g-1·h-1 | 85% | Spatially separate electron-hole pairs, Light absorption | 62 |
| CQD/OCN-x | Hydrothermal | CH4 | 1.73 μmol·g-1·h-1 | ─ | Charge separation and transfer, CO2 adsorption | 63 |
| NCD/LDH/CN | Hydrothermal | CH4 | 25.69 μmol·g-1·h-1 | 99% | Light absorption, Electron transfer, CO2 adsorption | 64 |
| CD-BVO/rGH | one-step in situ growth method | CO and CH4 | 61.54, 21.47 μmol·g-1·h-1 | ─ | Migration and separation of photoexcited carriers, Light absorption | 65 |
| CD/TOH | Ultrasound | CH4 | 26.8 μmol·g-1·h-1 | 98% | Light absorption, Electron transfer | 66 |
| CDs@CoOx | Calcination | CO | 8.1 μmol·g-1·h-1 | 89.3% | Electron transfer | 67 |
asynthesis method of composite materials |
| [1] |
|
| [2] |
( 刘雨菲, 张蜜, 路猛, 兰亚乾. 化学进展, 2023, 35(3): 349.)
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
( 刘鑫, 胡以怀, 王富伟. 炭素技术, 2020, 39(01): 12.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
( 孙海珠, 杨国夺, 杨柏. 高等学校化学学报, 2021, 42(2): 349.)
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
( 李程浩, 刘亚敏, 卢彬, 萨拉乌拉, 任先艳, 孙亚平. 化学进展, 2022, 34(3): 499.)
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
( 余家国. 新型太阳燃料光催化材料. 武汉: 武汉理工大学出版社, 2019. 11.)
|
| [34] |
( 朱永法, 姚文清, 宗瑞隆. 光催化:环境净化与绿色能源应用探索. 北京: 化学工业出版社, 2015. 3.)
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
/
| 〈 |
|
〉 |