

Rare Earth Mineral Processing Technology
Received date: 2023-08-25
Revised date: 2024-01-08
Online published: 2024-03-15
Supported by
National Key Research and Development Program of China(2022YFB3504302)
Opening Foundation of State Key Laboratory of Baiyunobo Rare Earth Resource Researches and Comprehensive Utilization(2020Z2117)
Science and Technology Service Network Initiative of Fujian(2022T3011)
rare earths have excellent physicochemical properties and have important applications in a wide range of fields,and always been recognized as a key mineral resource by many major countries,including China,the United States,Japan and Australia.However,rare earth raw minerals are diverse,low grade,often closely coexist with gangue minerals of similar nature,and its beneficiation and enrichment relies heavily on the development of the reagents on rare earth mineral processing technology.In This review,the research and development status of mineral-based rare earth ore flotation reagents,including collecting agents(hydroxamic acid,fatty acid,phosphoric acid and others),foaming agents(2#oil and others),modifying agents(depressants,activators)and their flotation mechanism are described.the innovation of chemical mineral processing reagents for ion-adsorption type rare earths ores,including leaching agents,and precipitation regents are summarized.In particular,this review pays special attention to the rare earth flotation collectors,especially for hydroxamic acid which is the current mainstream collector,and this review also provides a more detailed description of the capture mechanism of hydroxamic acid as well as the synthesis route.and at the end of this review,it also points out the future research direction of the reagents on rare earth mineral processing technology should be toward the higher selectivity,higher recovery,and more environmental friendliness.this review provides some useful reference for enterprises or personnel who are engaged in the rare earth mineral processing or related pharmaceuticals。
1 Introduction
2 Collecting agents on rare earth flotation
2.1 Hydroxamic acid
2.2 Fatty acid
2.3 Phosphoric acid
2.4 Other collectors
3 Foaming agents
4 Modifying agents
4.1 Depressants
4.2 Activators
5 Reagents of chemical beneficiation for Ion-adsorption type rare earth ore
5.1 Leaching agents
5.2 precipitation agents
6 Conclusion and outlook
Meiying Xie , Fan Yang , Liyan Xue , Kaixian Wang , Zhengming Jiang , Yazhu Li . Rare Earth Mineral Processing Technology[J]. Progress in Chemistry, 2024 , 36(5) : 741 -756 . DOI: 10.7536/PC230821
表1 Study on Rare Earth Flotation Performance of Partial Hydroxamic Acid CollectorTable 1 Study of rare earth flotation performance of some hydroxamic acids |
| Collector | Raw material | Flotation reagents | Concentrate |
|---|---|---|---|
| C7-C9 hydroxamic acid[19] | Rare earth ore from Australia with 19.13% rare earth oxide grade (REO). It mainly contains bastnaesite, monazite, limonite, magnetite and hematite. | Primary flotation: C7-C9 hydroxamic acid 1 kg/t, NaOH 1 kg/t, water glass 6.4 kg/t, humic acid 0.2 kg/t, sodium fluosilicate 1 kg/t, NaS 0.7 kg/t. | After a rough flotation, rare earth concentrate with 30.30% REO and 32.46% recovery was obtained. |
| H203、H205[20] | Rare earth ore from Bao steel processing plant (China) with 11.20% REO. It mainly contains bastnaesite, monazite, limonite, and silicate minerals. | Primary flotation: H203 or H205 4.0 kg/t, water glass 4.0 kg/t, XJ-01 foaming agent 0.048 kg/t, temperature 36~38 ℃. | After a rough flotation, H203 yields a rare earth concentrate with 31.54% REO and 79.05% recovery; H205 yields a rare earth concentrate with 36.77% REO and 79.40% recovery. |
| L102[21] | Rare earth ore from Bao steel processing plant (China) with 22.80% REO. It mainly contains bastnaesite, monazite, Iron minerals, fluorite, sodium pyroxene, sodium amphibole, and dolomite. | Primary flotation: Salicylic acid 2.85 kg/t, water glass 3.30 kg/t, 2# oil 0.076 kg/t, pulp concentration 45~50%, pH=8.5~9.5, temperature 40 ℃. | After "one rough and two fine" closed circuit flotation, a rare earth concentrate with 63.50% REO and 56.32% recovery was obtained. |
| H205、 H316[15] | Rare earth ore from Bao steel processing plant (China) with 10.10% REO. It mainly contains bastnaesite, monazite, Fluorite, apatite, hematite, pyrite, feldspar, quartz, mica, etc. | Bao steel ore dressing plant dilution workshop "one rough two fine" closed-circuit industrial flotation, the daily capacity of 465 t, the colectort was H316, sodium silicate as inhibitors, and H103 used as foaming agent. | After "one rough and two fine" flotation, H205 yields a rare earth concentrate with 53.09% REO and 67.11% recovery; H316 yields a rare earth concentrate with 53.31%; REO and 72.98% recovery. |
| P8[22] | Rare earth ore from Baotou (China) with 9.60% REO. It mainly contains fluorite, hematite, bastnaesite, sodium pyroxene, sodium amphibole, monazite and barite. | Primary flotation: P8 (the main component is H205) 2.4 kg/t; water glass 5.6 kg/t; turpentine oil 0.36 kg/t; temperature 65 ℃, concentration 30 wt%, pH=8~9. | After "one rough, three fine and one sweep" flotation, a rare earth concentrate with 50.3% REO and 78.6% recovery was obtained. |
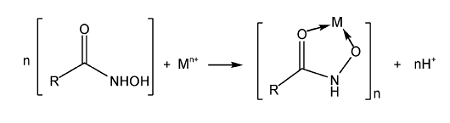
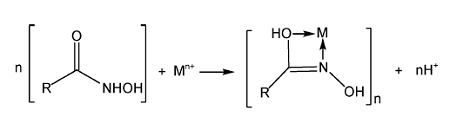
图3 部分脂肪酸捕收剂主要成分结构Fig. 3 Structure of the main components of some fatty acid collectors |
表2 Study on Flotation Performance of Some Fatty Acid CollectorsTable 2 Study of rare earth flotation performance of some fatty acid collectors |
| Collector | Raw material | Flotation reagents | Concentrate |
|---|---|---|---|
| sodium oleate [50] | Rare earth ore from an Australian processing plant with 1.07% REO. It mainly contains bastnaesite, monazite, quartz, mica, micas, magnetite and hematite. | Primary flotation: Sodium oleate 3.0 kg/t, water glass 1.5 kg/t, starch 1.5 kg/t. | After "one rough, one sweep and one concentrate" flotation, a rare earth concentrate with REO 2.25% and 63% recovery was obtained. |
| sodium oleate [51] | Rare earth ore from a processing plant in Bayan Obo (China) with 9.35% REO and CaF2 grade 26.49%. It mainly contains bastnaesite, monazite, Fluorite, hematite, magnetite, pyroxene, amphibole, quartz and feldspar. | Flotation of rare earths: SR (mixture of hydroxamic acid and Sodium Carbonate) as the collector. Primary flotation of fluorite: Sodium oleate 0.6 kg/t, acidic water glass 1.4 kg/t, SY (mixture of sodium hexametaphosphate and tannic acid) 1.28 kg/t. | After “rare earth flotation - fluorite pre-selection - fluorite selection - strong magnetic separation”, it can obtain rare earth concentrate with 50.54% REO and 92.32% recovery; and fluorite concentrate with 95.51% CaF2 grade and 50.98% recovery. |
| Tarr Oil [42] | Rare earth ore from Muntin Pass Rare Earth Mine (USA) with 7.7% REO. It mainly contains bastnaesite, Calcite, barite, quartz, etc. | Flotation process at the rare earth ore dressing plant in Muntin Pass, USA, with Tar oil as a collector and lignosulfonic acid as a depressant. | Rare earth concentrate with REO 65% and 80% recovery was obtained. |
| phthalic acid [52] | Fluorite concentrate from a processing plant in Bayan Obo (China) with a grade of 86.02% CaF2 and 5.72% REO. t mainly contains fluorite, bastnaesite, Calcite, ilmenite, quartz, etc. | Primary flotation: Phthalic acid 2.4 kg/t, water glass 2 kg/t, pH=5. | After "one rough and three fine" closed-circuit flotation, the fluorite concentrate with a grade of 95.12% CaF2 and a recovery of 84.05% was obtained. |
| phthalic acid [53] | Baotou mixed rare earth concentrates: bastnaesite (REO 51.18%, purity 79.99%), monazite (REO 12.80%, purity 20.01%), and it also contains fluorite and barite. | Primary flotation: Phthalic acid 2.2 kg/t, alum as depressant 4.0 kg/t. | After "one rough and two sweeps" bastnaesite concentrate (REO 68.81%, purity 95.04%) and monazite concentrate (REO 58.55%, purity 95.34%) were obtained. |
表3 Study on Flotation Performance of Some Phosphoric Acid CollectorsTable 3 Study of rare earth flotation performance of some phosphonic acid collectors |
| Collector | Raw material | Flotation reagents | Concentrate |
|---|---|---|---|
| Styrylphosphonic acid [60] | Shandong Huishan (China) rare earth ore with 6.10% REO. It mainly contains bastnaesite, Parisite, hematite, limonite. | Primary flotation: Sulfuric acid 0.5 kg/t, kerosene 2.5 kg/t, Styrophylphosphonic acid 1.5 kg/t, pine oil 0.2 kg/t. | After "One rough and two fine" open circuit flotation, a rare earth concentrate with 60.13% REO and 48.36% recovery was obtained. |
| Styrene phosphonic acid, toluene phosphonic acid, alpha-hydroxybenzylphosphonic acid, benzylphosphonic acid, (1-hydroxy-1,3- dimethyl) butylphosphonic acid[46] | the same as above | Different collectors use different optimal flotation conditions. | Rare earth concentrates were obtained: styrylphosphonic acid (REO 41.67%, recovery 50.50%); toluene phosphonic acid (REO 43.23%, recovery 37.29%); α-hydroxybenzylphosphonic acid (REO 55.55%, recovery 66.35%); benzylphosp- honic acid (REO 34.39%, recovery 44.05%); and (1-hydroxy -1,3- dimethyl) butylphosphonic acid (REO 33.77%, recovery 53.69%). |
| P538[61] | Baotou mixed rare earth concentrates: REO > 68%, bastnaesite 71.67%, Monazite 28.33%. | Multi-stage flotation, P538 as collector added in batches, rough flotation reagent was: citric acid 200 mg/L, frothing agent MIBC 12 mg/L, pH=5.0. | Bastnaesite concentrate with purity of 91% and recovery of 70.18% and monazite concentrate with purity of 61.2% and recovery of 73.18% were obtained. |
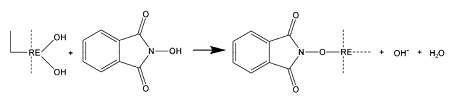
表4 Some other reported rare earth flotation depressantsTable 4 Other reported flotation depressants of rare earth |
| depressant | Inhibited Mineral | Possible Mechanisms of Inhibition | disadvantages | |
|---|---|---|---|---|
| Alum [86,87] | It has good inhibition effect on barite, fluorite, calcite and other barium calcium salt minerals. And it can also be used to inhibit monazite when separating bastnaesite and monazite. | Alum hydrolyzed Al3+ preferentially combines with SO42- on the surface of barite to inhibit barite. Ca2+ on the surface of calcite and fluorite preferentially combines with AlO2- and SO42- hydrolyzed by alum to inhibit calcite and fluorite. | Bastnaesite will be partially inhibited when alum used in excess. | |
| Lignosulfonate [86,87] | It can effectively inhibit barite and calcite, and there is little difference in the inhibition effect under high temperature conditions. | It contains phenolic hydroxyl functional groups, can be selectively adsorbed on the surface of barite and calcite to make the mineral surface hydrophilic. | Needs to control the dosage, overuse is not conducive to the flotation of rare earth ores, such as bastnaesite. | |
| Sodium fluorosilicate [86] | It can be used to inhibit barite, calcite, fluorite, quartz, feldspar, and other silicates. | Hydrolyzed SiF62- continues to be hydrolyzed to SiO2 micelles adsorbed on the mineral surface making the mineral hydrophilic. Hydrolyzed SiF62-continues to be hydrolyzed to HF, which dissolves the dissolved feldspar surface to generate free H2SiO3 micelles, preventing the capture of the trapper. It can preferentially desorb fatty acid-based traps from the surface of vein minerals. | It results in a lower pH of the slurry, requiring the addition of more pH adjusters such as sodium carbonate. | |
| Sodium hexametaphosphate [54,57,88] | It can be used to inhibit calcite and barite. | Hydrolyzed Na4P6O182- forms hydrophilic and stable complexes with Ca2+ on the mineral surface in the slurry. | Has a strong inhibition effect on Bastnaesite, so it needs to strictly control the dosage | |
| Sodium sulfide [89] | It can be act as inhibitors of zircon and activators of monazite when fatty acid as collector | Hydrolyzed S2- and HS- ions are adsorbed on the surface of zircon, preventing the adsorption of collectors on zircon or leading to selective resolution of collectors such as oleic acid on the surface of zircon. | Easily releases toxic hydrogen sulfide gas in the air. | |
| Citrate [61] | It can inhibit fluorite and aluminum silicate minerals such as calcite and mica. And it can also be used to inhibit bastnaesite when separating monazite and bastnaesite in flotation. | Citric acid and mineral lattice surface cations have a strong ability to cooperate with the adsorption on the surface of the inhibited minerals, and forming hydrophilic chelates to hinder the adsorption of the collectors on the gangue minerals. Citric acid has a greater ability to dissolve cerium fluorocarbon, and the mineral surface loses more active centers and is subsequently inhibited. | High acidity will reduce the pH of the slurry, and dosage also needs to be controlled. | |
| Starch, modified starch [90⇓-92] | It can be used to inhibit rutile, ilmenite, zircon, hematite, quartz and so on. | Polar functional groups such as hydroxyl groups on the molecular chain are adsorbed on the mineral surface through hydrogen bonding, electrostatic and chelating effects, and are inhibited by the formation of a hydrophilic adsorption layer on the mineral surface. | Natural starch has poor water solubility and poor selective adsorption capacity on mineral surfaces. Modified starch has small applicability, and mostly stays in the laboratory research stage. | |
| EDTA[93⇓-95] | It is mainly used to inhibit calcium-containing minerals such as fluorite and calcite. Low doses can remove Ca2+ ions from the surface of monazite and realize the activation of monazite, but it also has a certain inhibition effect on monazite in large doses. | Complexation with dissolved Ca2+ on the mineral surface hinders the adsorption of collectors such as hydroxamic acid on the surface of minerals such as fluorite. | Dosage needs to be controlled and often requires synergistic use with other inhibitors. | |
| CMC[86,96] | It can be used to inhibit silicate minerals and minerals containing calcium and magnesium. | After hydrolysis, carboxymethyl anions are electrostatically attracted to the cations on the mineral surface, carboxyl groups form a water film with water through hydrogen bonding, and part of the CMC is adsorbed on the mineral surface as negatively charged micelles, which ultimately results in the hydrophilicity of the mineral surface and its inhibition. | Requires synergistic use with other inhibitors. | |
| Guar gum [97] | Inhibits minerals such as talc and silicates | Similar to starch and modified starch inhibition mechanism | It is easy to cause the slurry to become viscous, reduce the flotation grade, so it needs to strictly control the dosage. | |
表5 Test Results of Leaching of an Ionic Rare Earth Ore with Partial Reagents[106]Table 5 leaching results of some Leaching reagents on an ion-adsorption type rare earths ores[106] |
| Leaching agent | Concentration | pH | Leaching rate of RE /% | Leaching agent | Concentration | pH | Leaching rate of RE /% |
|---|---|---|---|---|---|---|---|
| HCl | 2% | 0.5 | 52.92 | KCl | 1 mol/L | 5.4 | 92.99 |
| H2SO4 | 2% | 0.5 | 76.09 | Ammonium citrate | 0.5 mol/L | 4.5 | 95.18 |
| NH4Cl | 1 mol/L | 5 | 94.72 | Fe2(SO4)3 | 1% | 2.5 | 70.00 |
| CH3COONH4 | 1 mol/L | 6 | 94.66 | FeSO4 | 1% | 2.5 | 67.00 |
| NaCl | 1 mol/L | 5.4 | 97.53 | (NH4)2SO4 | 2% | 4.5 | 98.50 |
| [1] |
( 李文昌, 李建威, 谢桂青, 张向飞, 刘洪. 地学前缘, 2022, 1(29): 1.)
|
| [2] |
( 何宏平, 杨武斌. 大地构造与成矿学, 2022, 5(46): 829.)
|
| [3] |
|
| [4] |
(黄礼煌. 稀土提取技术. 北京: 冶金工业出版社, 2006.
|
| [5] |
|
| [6] |
( 张博, 宁阳坤, 曹飞, 杨卉芃. 矿产综合利用, 2018, 4: 7.)
|
| [7] |
|
| [8] |
|
| [9] |
( 李恩泽, 杜志平, 王波, 成怀刚, 程芳琴. 化学进展, 2016, 28: 1417.)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
( 任俊. 有色金属, 1998, 2(50): 36.)
|
| [16] |
( 缪贺成. 内蒙古科技大学硕士论文, 2021.)
|
| [17] |
( 李娜, 秦玉芳, 王其伟. 矿冶工程, 2021, 6(41): 43.)
|
| [18] |
( 彭蓉, 魏志聪, 刘洋, 白睿, 王衡嵩, 柏少军. 有色金属工程, 2021, 1(11): 73.)
|
| [19] |
( 何晓娟, 饶金山, 罗传胜, 陈志强, 刘超, 张军. 中国稀土学报, 2016, 34(2): 244.)
|
| [20] |
( 徐金球, 徐晓军, 王景伟. 有色金属, 2002, 3(54): 72.)
|
| [21] |
( 任皞, 胡永平. 金属矿山, 1996, 11: 20.)
|
| [22] |
( 杨治仁. 东北大学博士论文, 2017.)
|
| [23] |
( 唐清. 中南大学硕士论文, 2014.)
|
| [24] |
( 刘子恩, 吴红, 杜文涛. 化工中间体, 2012, 9(7): 43.)
|
| [25] |
|
| [26] |
( 黄林旋. 矿产综合利用, 1980, 1: 93.)
|
| [27] |
( 周礼义. 中南大学硕士论文, 2010.)
|
| [28] |
( 唐清, 钟宏, 王帅, 彭静. 化工进展, 2014, 33(3): 703.)
|
| [29] |
|
| [30] |
( 高颖剑, 林江顺. 国外金属矿选矿, 1997, 34(6): 54.)
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
( 王淀佐. 浮选剂作用原理及应用. 北京: 冶金工业出版社, 1982.)
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
( 罗家珂, 任俊, 唐芳琼, 周高云. 中国稀土学报, 2002, 5(20): 385.)
|
| [44] |
|
| [45] |
( 兰玉成, 徐雪芳, 黄风兰, 赵其华. 稀土, 1983, 4: 27.)
|
| [46] |
( 张泾生, 阙煊兰, 见百熙. 有色金属, 1982, 2(34): 29.)
|
| [47] |
|
| [48] |
( 王鑫, 王亚运, 于传兵, 康金星. 中国矿山工程, 2020, 4(49): 73.)
|
| [49] |
|
| [50] |
|
| [51] |
( 王丽明, 李宏静, 白春霞, 睢月婷, 刘涛. 矿产保护与利用, 2022, 6: 52.)
|
| [52] |
|
| [53] |
( 宋常青. 有色金属(选矿部分), 1993, 04: 5.)
|
| [54] |
(龚明光. 泡沫浮选. 北京: 冶金工业出版社, 2007.)
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
( 张泾生, 见百熙. 矿冶工程, 1981, 02: 26.)
|
| [61] |
( 周高云, 罗家珂. 有色金属, 1989, 4(41): 33.)
|
| [62] |
( 周高云, 罗家珂. 中国稀土学报, 1990, 3(8): 261.)
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
( 任俊. 金属学报, 1993, 29(6): 49.)
|
| [67] |
( 古映莹, 朱玉霜.
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
( 张晓艳, 李梅, 高凯, 马林林, 段海军, 李琅琅. 稀有金属, 2019, 1.)
|
| [73] |
|
| [74] |
( 李燊毅, 刘兴平, 蒋之飞, 陈松, 任浏祎, 包申旭. 金属矿山, 2022, 6: 115.)
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
( 张伟, 李解, 胡庆成, 康德伟, 许晓乐. 稀土, 2021, 1(42): 87.)
|
| [81] |
( 王斯日古楞. 现代矿业, 2016, 3(9): 116.)
|
| [82] |
( 姚志明, 宋传兵, 张齐. 金属矿山, 2014, 32(9): 39.)
|
| [83] |
|
| [84] |
( 梁经冬, 谢士埕, 杨乃庚, 商维君. 湖南冶金, 1986, 1: 14.)
|
| [85] |
( 林一明, 王维清, 蒋颖, 陈城, 朱欣宇, 曹轩. 稀土, 2020, 3(41): 78.)
|
| [86] |
( 池汝安, 王淀佐. 稀土矿物加工. 北京: 科学出版社, 2014.)
|
| [87] |
( 朱智慧. 内蒙古科技大学硕士论文, 2019.)
|
| [88] |
( 王成行, 时晗, 邱显扬, 胡真. 中国稀土学报, 2020, 6(38): 816.)
|
| [89] |
|
| [90] |
|
| [91] |
( 姜志学, 刘志国, 王鑫. 现代矿业, 2019, 35(11): 168.)
|
| [92] |
( 田付强, 李亚超, 曹亦俊, 范桂侠. 矿产保护与利用, 2022, 01: 82.)
|
| [93] |
|
| [94] |
( 王介良, 迈倩琳, 程泽宇, 曹钊. 有色金属(选矿部分), 2021, 06: 181.)
|
| [95] |
( 吴旭, 张艳清, 曹钊. 矿产保护与利用, 2022, 3: 1.)
|
| [96] |
( 任俊. 稀土, 1990, 61(2): 59.)
|
| [97] |
|
| [98] |
( 罗家珂, 陈祥涌. 中国稀土学报, 1985, 3(3): 7.)
|
| [99] |
( 迈倩琳. 内蒙古科技大学硕士论文, 2021.)
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
( 李健民, 刘殿文, 孙运礼, 郭海宁. 稀土, 2017, 38(5): 120.)
|
| [105] |
( 余永富, 王采辉, 李养正, 罗积扬. 矿冶工程, 1984, 4(4): 29. )
|
| [106] |
( 黄礼煌. 稀土提取技术. 北京: 冶金工业出版社, 2006. 144.)
|
| [107] |
( 高志强, 周启星. 生态学杂志, 2011, 30(12): 2915.)
|
| [108] |
|
| [109] |
( 王瑞祥, 谢博毅, 余攀, 张兆雪, 毛继勇, 熊家春. 稀有金属, 2015, 39(11): 1060.)
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
( 李琼, 何正艳, 张臻悦, 张婷婷, 钟诚斌, 池汝安. 稀土, 2015, 1(36): 18.)
|
| [115] |
( 邱廷省, 罗仙平, 方夕辉, 胡玖林, 成先雄, 郝志伟. 矿产综合利用, 2002, 5: 14.)
|
| [116] |
( 唐学昆, 田君, 尹敬群, 罗仙平. 有色金属科学与工程, 2013, 4(2): 85.)
|
| [117] |
( 周芳, 冯健, 池汝安. 中国稀土学会2021学术年会论文摘要集, 2021, 44.)
|
| [118] |
|
| [119] |
( 姚慧琴, 欧阳克氙, 饶国华. 江西科学, 2005, 6(23): 721.)
|
| [120] |
( 冉秀川. 武汉理工大学硕士论文, 2018.)
|
| [121] |
( 黄小卫, 于瀛, 冯宗玉, 赵娜. 中国专利. CN102190325B. 2014.)
|
| [122] |
|
| [123] |
( 孙东江, 王志勇. 中国专利. CN104611561A. 2015. )
|
| [124] |
( 许瑞高, 钟化云, 李早发. 中国专利. CN103436720B. 2015.)
|
| [125] |
( 黄小卫, 肖燕飞, 冯宗玉, 陈迎迎, 董金诗, 黄莉, 王良士. 中国专利. CN105861828B. 2018.)
|
| [126] |
( 陈久昌, 姚清霞, 邱建民, 吕英威, 刘庆生. 中国稀土学报, 2020, (38): 646.)
|
| [127] |
|
| [128] |
( 黄金. 世界有色金属, 2018, 242.)
|
| [129] |
( 余攀. 江西理工大学硕士论文, 2014.)
|
| [130] |
|
| [131] |
( 高国华, 赖富国, 徐耗祥, 张乾, 郭浩, 肖燕飞. 稀有金属, 2019, 43(4): 409.)
|
| [132] |
( 孟祥龙, 冯宗玉, 黄小卫, 董金诗, 王良士, 赵龙胜. 稀有金属, 2018, 4(42): 409.)
|
| [133] |
( 黄日平, 邬元旭, 钟月明, 凌卫东, 陈勇春, 罗志强, 邬元斌, 徐欣. 中国专利. CN101475202. 2009.)
|
/
| 〈 |
|
〉 |