

The Electronic Principle of Nanomaterial Surface Chemistry
Received date: 2024-01-08
Revised date: 2024-03-11
Online published: 2024-03-18
Supported by
National Natural Science Foundation of China(21801012)
Revealing The intrinsic electronic principles driving the surface chemistry of nanomaterials is a central goal in nanoscience;however,the concepts and theoretical frameworks have long remained incomplete and unsystematic.this review systematically introduces a theoretical framework to reveal the interaction mechanisms and trends of surface ligands with nanomaterials at the electronic level,on the basis of competitive orbital redistribution in chemisorption and a concept of orbital potential,the characteristic electronic attribute directly determining surface reactivity.Based on the competitive interactions between surface coordination bonds and bulk energy bands,This theoretical framework can provide coherent answers to these key scientific issues.(1)the opposite and uniform relation of surface activity and stability in nanomaterials originates from the normalization principle of wavefunctions.(2)the physical nature of enhanced surface activity by size reduction lies in two mechanisms:weakening the constrain strength to surface valence atomic orbitals by nanomaterial energy bands,and amplifying the effects of other structural parameters like defects.(3)Nanoscale cooperative chemisorption(NCC)model generally reveals the electronic-level mechanisms and common rules how ligand coverage regulates the energy band states and physical/chemical properties of nanomaterials.(4)the roles and interaction mechanisms of nanomaterial size(r),specific surface area(S/V),surface ligands,and ligand coverage(θ)in nanomaterial surface chemical reactions are elucidated.
1 Introduction
2 Nanomaterial surface chemistry
2.1 Key science issues
2.2 Three types of understanding viewpoints
2.3 Nanomaterial surface coordination chemistry
2.4 Four modes of nanomaterial surface effects
3 Electronic principle of structure-function relationships
3.1 Structure-function relationship in physical science
3.2 Electronic attributes
3.3 Quantum size effect
4 Chemisorption model based on competitive orbital redistribution
4.1 Chemisorption interaction
4.2 Competitive redistribution of surface valence orbitals
4.3 Orbital potential
4.4 Structure-function relationship of surface reactivity
5 Electronic principle of size-dependent surface reactivity
5.1 Meaning of surface activity
5.2 Mathematic model of surface reactivity
5.3 Dual roles of size reduction in enhancing surface reactivity
6 Nanoscale competitive chemisorption model
6.1 Relationship of energy band and surface reactivity
6.2 Nanoscale competitive chemisorption model
6.3 The roles of r,S/V,andθin nanosurface chemistry
6.4 Two-electronic-state competition model
6.5 The uniform principle of ligand effect on photoluminescence
7 Comparison of typical adsorption models
7.1 Adsorption isotherm model
7.2 Electronic model of chemisorption
7.3 Chemisorption model of nanomaterial
8 Summary and outlook
Guolei Xiang . The Electronic Principle of Nanomaterial Surface Chemistry[J]. Progress in Chemistry, 2024 , 36(6) : 851 -866 . DOI: 10.7536/PC240105
图2 基于Green的共价键分类方法的三种配体类型。在带电中性时,L型配体共享2个电子属于路易斯碱,X型共享1个电子属于自由基,Z型配体提供0个电子为路易斯酸[32]Fig. 2 LXZ ligands based on Green’s Covalent Bond Classification (CBC) method. In their neutral forms, L-type ligands donate two electrons and are identified as Lewis bases, X-type ligands donate one electron and are radicals, and Z-type ligands donate 0 electron as Lewis acids[32] |
图4 电子结构状态的不同属性。电子结构是一个全局的整体状态,从不同的角度与层次看表现出不同的局部属性,要用不同的参数描述。每一属性往往是某种性能的直接决定机制Fig. 4 Attributes of electronic states. Electronic structure is the global state for a matter system, but it contains many different attributes when considered at different aspects. An attribute usually determines a certain property and function performance |
图6 基于表面价原子轨道(SVAO)竞争重构机制的化学吸附模型:(a)表面配位键的形成将活性中心原子的价轨道束缚在表面态,减小其往体相拓展参与能带的形成[50]。(b)化学吸附前后体系电子态变化示意图。左图为未发生相互作用的吸附质前线轨道及表面的能带电子态,此时SVAO主要往体相拓展参与能带的形成;右图为化学吸附后体系的组合电子态,一部分SVAO (fS)与吸附质的前线轨道重叠形成表面吸附键的成键态,另一部分SVAO (fB)与表面中周围其他原子的价轨道重叠并在晶格内拓展参与能带的形成Fig. 6 Chemisorption model based the competitive redistribution of surface valence atomic orbitals (SVAO). (a) The formation of surface coordination bond confines the valence orbitals of active center atoms into surface chemisorption states, reducing their extension into the bulk phase to participate in the formation of energy bands[50]. (b) Scheme showing the changes in electronic states before and after chemisorption. The left diagram illustrates the electronic states of the adsorbate's frontier orbitals and the surface energy bands without chemisorption. The right diagram shows the combined electronic states after chemisorption, in which a portion of SVAO (fS) overlaps with adsorbate's frontier orbitals to form the bonding states of chemisorption bonds, while other portion of SVAO (fB) overlaps with the valence orbitals of surrounding atoms in the surface phase to participate in the formation of energy bands |
图8 纳米尺度协同化学吸附过程(NCC)的电子结构变化机制与作用结果:(a)表面价原子轨道随配体覆盖度变化的分布趋势与协同重构机制。(b)表面配体调控纳米材料能带电子态与物理化学性质的物理化学图像与机制[65]Fig. 8 The mechanism of nanoscale cooperative chemisorption (NCC) in inducing changes in the electronic states of nanomaterials. (a) Redistribution trends of surface valence atomic orbitals depending on the coverage of surface ligands on nanomaterials. (b) The physical and chemical mechanism depicting how surface ligands regulate the nanomaterial's energy band states and physical and chemical properties[65] |
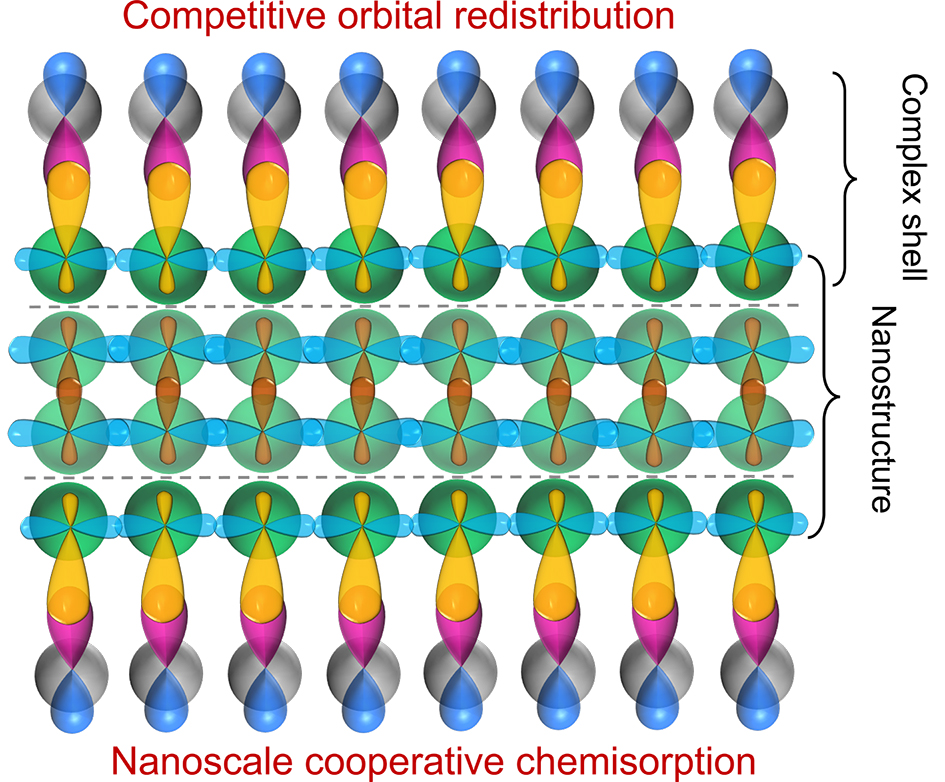
图9 轨道竞争重构产生的轨道分布层面的纳米材料“核壳”结构图示:(a) 轨道竞争重构与纳米尺度协同吸附机制将表面价原子轨道由能带态重构到表面吸附态,形成由最表层原子与配体组成的表面配位层和内部体相原子组成的内核。表层原子与内核原子间的轨道耦合作用减弱。(b) 球形纳米颗粒的轨道分布“核壳”结构图示Fig. 9 Schematic diagrams of orbital-level 'core-shell' structure of nanomaterials. (a) The polarization of surface valence atomic orbitals from band states to surface chemisorption state caused by competitive orbital redistribution and nanoscale cooperative chemisorption. This results in the formation of surface complex shell composed of outermost surface atoms and ligands, and the inner core composed of bulk atoms. The orbital couplings between surface atoms and core atoms are weakened. (b) Schematic 'core-shell' structure of spherical nanoparticles in orbital distribution |
图10 纳米材料的表面态荧光与体相能带态荧光图示。表面态光吸收与发射源于电子在表面吸附键的成键态和反键态间的跃迁,体相态光吸收与发射源于电子在能带态的价带和导带间的跃迁。表面配位层与内核层间的弱轨道耦合效应抑制了光生激子的扩散与捕获,从而提高两种发光过程的量子产率Fig. 10 Photoluminescence modes of ligand-capped nanomaterials resulting from surface chemisorption states and bulk energy band states. Optical absorption and emission of surface states result from the transitions of electrons between the bonding and antibonding states of surface bonds. The absorption and emission of band states arise from the transitions of electrons between the valence band and the conduction band. The weakened orbital couplings at the interfaces of surface complex shells and inner cores can suppress the bidirectional diffusions and capture of photogenerated excitons, thereby enhancing the quantum yields of both luminescent processes |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
(秦瑞轩, 邓果诚, 郑南峰. 化学进展, 2020, 32: 1140.)
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
/
| 〈 |
|
〉 |