

Covalent Organic Frameworks for Proton Exchange Membranes
Received date: 2023-05-30
Revised date: 2023-07-14
Online published: 2023-08-06
Supported by
National Natural Science Foundation of China(21975112)
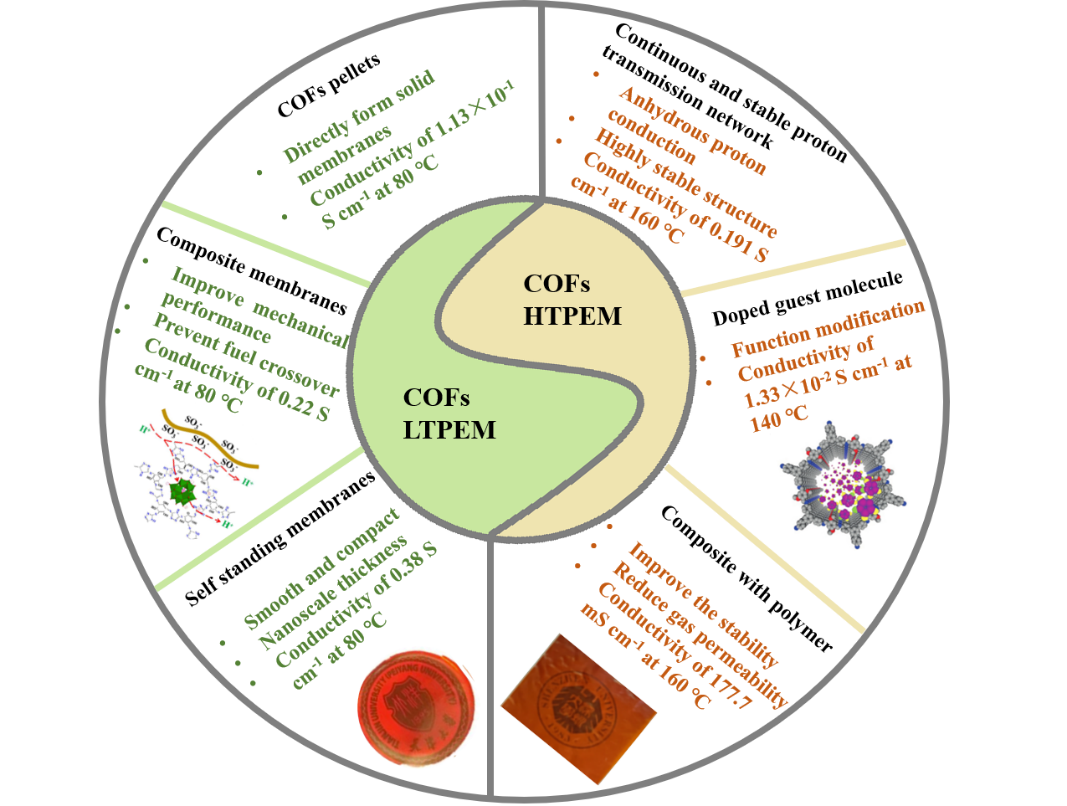
Covalent organic frameworks (COFs), as a new type of organic porous materials, are highly crystalline and orderly porous, exhibiting functional modifiability, structural tunability and high stability. The regular pore channels of COFs can accommodate a variety of proton carriers and proton donors to build continuous and stable proton transport channels, playing a great role in both aqueous and anhydrous proton conduction. The application of COFs to the field of proton exchange membranes is of great research significance and value. In this paper, the characteristics of different types of proton exchange membranes, such as COFs solid electrolyte membranes, polymer matrix-COFs composite membranes, COFs self-supporting membranes and the modification methods to improve the performance of COFs proton exchange membranes are summarized from the aspects of COFs as proton exchange membranes for low temperature fuel cells and high temperature fuel cells, respectively. The relevant representative research of COFs in the field of fuel cell proton exchange membranes in recent years is reviewed. Finally, the application prospects of COFs proton exchange membranes are discussed and prospected.
1 Introduction
2 Covalent organic frameworks
2.1 Structure of COFs
2.2 Synthesis of COFs and COFs membrane
2.3 Application of COFs
3 COFs fuel cell proton exchange membrane
3.1 COFs low-temperature fuel cell proton exchange membranes
3.2 COFs high-temperature fuel cell proton exchange membranes
4 Conclusion and outlook
Weiyu Zhang , Jie Li , Hong Li , Jiaqi Ji , Chenliang Gong , Sanyuan Ding . Covalent Organic Frameworks for Proton Exchange Membranes[J]. Progress in Chemistry, 2024 , 36(1) : 48 -66 . DOI: 10.7536/PC230529
图4 自下而上策略制备COFs膜 (a)溶剂热合成法[38] (b)层状自组装聚合法[44] (c)液-液界面聚合法[41]Fig. 4 Bottom-up strategies for preparing COFs membranes (a) Solvothermal synthesis[38] (b) Laminar assembly polymerization[44] (c) Interfacial polymerization[41]. Ref 38, Copyright 2011, American Association for the Advancement of Science; Ref 44, Copyright 2019, American Association for the Advancement of Science; Ref 41, Copyright 2023, American Chemical Society |
图12 (a)IPC-COF膜组装和孔隙结构示意图;(b)IPC-COF的SEM图像;(c)IPC-COF的XRD图像;(d)IPC-COF膜和文献中报道的PEMs的溶胀率与IEC之间的关系[76]Fig. 12 (a)Schematic illustration of IPC-COF membrane assembly and pore structures; (b)SEM image of IPC-COF; (c)XRD pattern of IPC-COF; (d)Swelling ratio versus IEC value from IPC-COF membrane and existing PEMs as reported in the literature[76]. Copyright 2020, Wiley-VCH |
表1 不同COFs质子交换膜的性能Table 1 Properties of different COFs proton exchange membranes |
| COFs | Sample type | Thermal decomposition temperature (℃) | Tensile strength(MPa) | Activation energy (kJ·mol-1) | σ (S·cm-1) | Maximum power density (mW·cm-2) | ref |
|---|---|---|---|---|---|---|---|
| PA@aza-COF-2 | Pellet | 250 | — | 45 | 4.8×10-3 (50°C, 97% RH) | — | 64 |
| NSU-10(R) | Pellet | — | — | — | 3.96×10-2 (room temperature, 97% RH) | — | 65 |
| H3PO4@NKCOF-1 | Pellet | 260 | — | 14 | 1.13×10-1 (80 ℃, 98% RH) | 81 (60°C) | 66 |
| EB-COF:PW12 | Pellet | 300~400 | — | 24 | 3.32×10-3 (25 ℃, 97% RH) | — | 67 |
| Nafion/H3PO4@S1-15 | Composite membrane | 389 | — | — | 6.04×10-2 (80 ℃, 51% RH) | 277.8 (60°C) | 71 |
| Nafion/Z-COF-10 | Composite membrane | 359.5 | 25~28 | 7.7 | 2.2×10-1 (80 ℃, 100% RH) | — | 72 |
| Nafion-SCONs-0.6 | Composite membrane | 280~350 | 27.3±0.4 | — | 2.65×10-1 (80 ℃) | 118.2 (80°C) | 73 |
| SPEEK/HPW@COF-15 | Composite membrane | 250~360 | 75.0 | 23.76 | 6.2×10-3 (65 ℃, 40% RH) | — | 74 |
| IPC-COF | Membrane | — | 91.2±6 | 12 | 3.8×10-1 (80 ℃, 98% RH) | 1100 (80°C) | 76 |
| SPC-COF-NS | Membrane | — | 24.3 | — | 3.64×10-1 (80 ℃) | 891.7 (80°C) | 77 |
| SCOF | Membrane | — | — | 19 | 5.4×10-1 (80 ℃) | — | 75 |
图15 (a)TPB-DMeTP-COF的合成路线;(b)TPB-DMeTP- COF的单个六边形大环结构;(c)TPB-DMeTP-COF单个六边形通道结构(灰色:C原子;绿色:N原子;省略了CH3和H单元);(d)77 K下TPB-DMeTP-COF的氮气吸附等温线(圆圈:吸附;三角形:解吸)[83]Fig. 15 (a)The synthesis of TPB-DMeTP-COF; (b)The structure of one hexagonal macrocycle; (c)The structure of a 1D channel (grey, C;green, N;CH3 units and H are omitted for clarity); (d)Nitrogen sorption isotherms of TPB-DMeTP-COF measured at 77 K (circle, adsorption; triangle, desorption)[83]. Copyright 2020, Spring Nature |
图16 (a)TPT-COF的合成;(b)反平行堆积模型下TPT-COF相应细化晶体结构的俯视图和侧视图(灰色、蓝色和白色球体分别代表C、N和H原子);(c)TPT-COF的PXRD谱图[84]Fig. 16 (a)The synthesis of TPT-COF; (b)Top and side views of the corresponding refined crystal structures of TPT-COF with the antiparallel stacking model (gray, blue, and white spheres represent C, N, and H atoms, respectively); (c)The PXRD patterns of TPT-COF[84]. Copyright 2022, Wiley-VCH |
表2 不同高温COFs质子交换膜的主要性能Table 2 Properties of different COFs HTPEMs |
| COFs | Sample type | Thermal decomposition temperature (°C) | Tensile strength(MPa) | Activation energy (kJ·mol-1) | σ (S·cm-1) | Maximum power density (mW·cm-2) | ref |
|---|---|---|---|---|---|---|---|
| H3PO4@TPB-DMeTP-COF | Pellet | — | — | 34 | 1.91×10-1 (160 ℃, 0 RH) | — | 83 |
| TPT-COF | Pellet | 525 | — | 17 | 1.27×10-2 (160 ℃, 0 RH) | — | 84 |
| H3PO4@TPB-DABI-COF | Pellet | — | — | 17 | 1.52×10-1 (160 ℃, 0 RH) | — | 85 |
| im@TPB-DMTP-COF | Pellet | 220 | — | 25 | 4.37×10-3 (130 ℃, 0 RH) | — | 59 |
| PIL-TB-COF | Pellet | 221 | — | 30 | 2.21×10-3 (120 ℃, 0 RH) | — | 87 |
| F6-[dema]HSO4-1.5 | Pellet | 300 | — | 34 | 1.33×10-2 (140 ℃, 0 RH) | — | 88 |
| 30%-CTFs-OPBI | Membrane | 300 | 7.7 | — | 7.71×10-2 (160 ℃, 0 RH) | 534.3 | 92 |
| 40%-COF-OPBI | Membrane | 300~400 | 12.2 | — | 1.777×10-1 (160 ℃, 0 RH) | 774.7 | 93 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
(贺高红, 焉晓明, 吴雪梅, 胡正文, 杜立广. 膜科学与技术, 2011, 31(3): 140.).
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
(孙鹏, 李忠芳, 王传刚, 王燕, 崔伟慧, 裴洪昌, 尹晓燕. 材料工程, 2021, 49(1): 23.).
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
/
| 〈 |
|
〉 |