

Applications of Metallomics and Metalloproteomics Techniques in Biomedical Research
Received date: 2023-03-07
Revised date: 2023-04-09
Online published: 2023-06-12
Supported by
Fundamental Research Funds for the Central Universities(JQN2022026)
Metals are recognized as essential cofactors in life processes and are fundamental elements in many key cellular processes. Metallomics, as an emerging research field, aims to understand and reveal the functions of bio-active metals and the molecular mechanisms of metal-based life processes, and the related studies have received growing attention due to its rapid development as a frontier science. In this review, we first introduce the concept of metallomics and the related research technologies, and focuses on an important research branch in this field, metalloproteomics, which aims to recognize the relationships between biometals and cellular proteins in a systematic manner. The development of this field has provided a number of practical research tools. We summarize and highlight the recent applications, major progress and important research findings of metallomics and metalloproteomics in biomedical research, which cover the studies of metals/metallodrugs uptake at the single-cell level, the distributions of metals/metallodrugs in cells, tissues and organs, the identification and characterization of intracellular metal-binding proteins, as well as the bioinformatics analysis of metalloproteins. Based on the current research status, the challenges and prospects of the applications of metallomics techniques in biomedical research are further discussed. Moreover, popularization of the metalloproteomics research would be an innovate and efficient way to obtain a complete understanding of the role of bioactive metals in cells. We believe that the development of new methodologies in metallomics and metalloproteomics, as well as the discovery of novel metal-related biological mechanisms will facilitate, support and expand the research perspectives in biomedicine and clinical research.
1 Introduction
2 Metallomics and metalloproteomics
2.1 Definition
2.2 Research methods and techniques
3 Applications and progress in biomedical research
3.1 Metals/metallodrugs uptake studies at single cell levels
3.2 Distribution studies of metals/metallodrugs in cells and tissues
3.3 Identification of metallodrug-targeting proteins in cells
4 Conclusion and outlook
Key words: metallomics; metalloproteomics; metallodrugs; drug-targeting proteins
Yuchuan Wang . Applications of Metallomics and Metalloproteomics Techniques in Biomedical Research[J]. Progress in Chemistry, 2023 , 35(10) : 1492 -1504 . DOI: 10.7536/PC230301
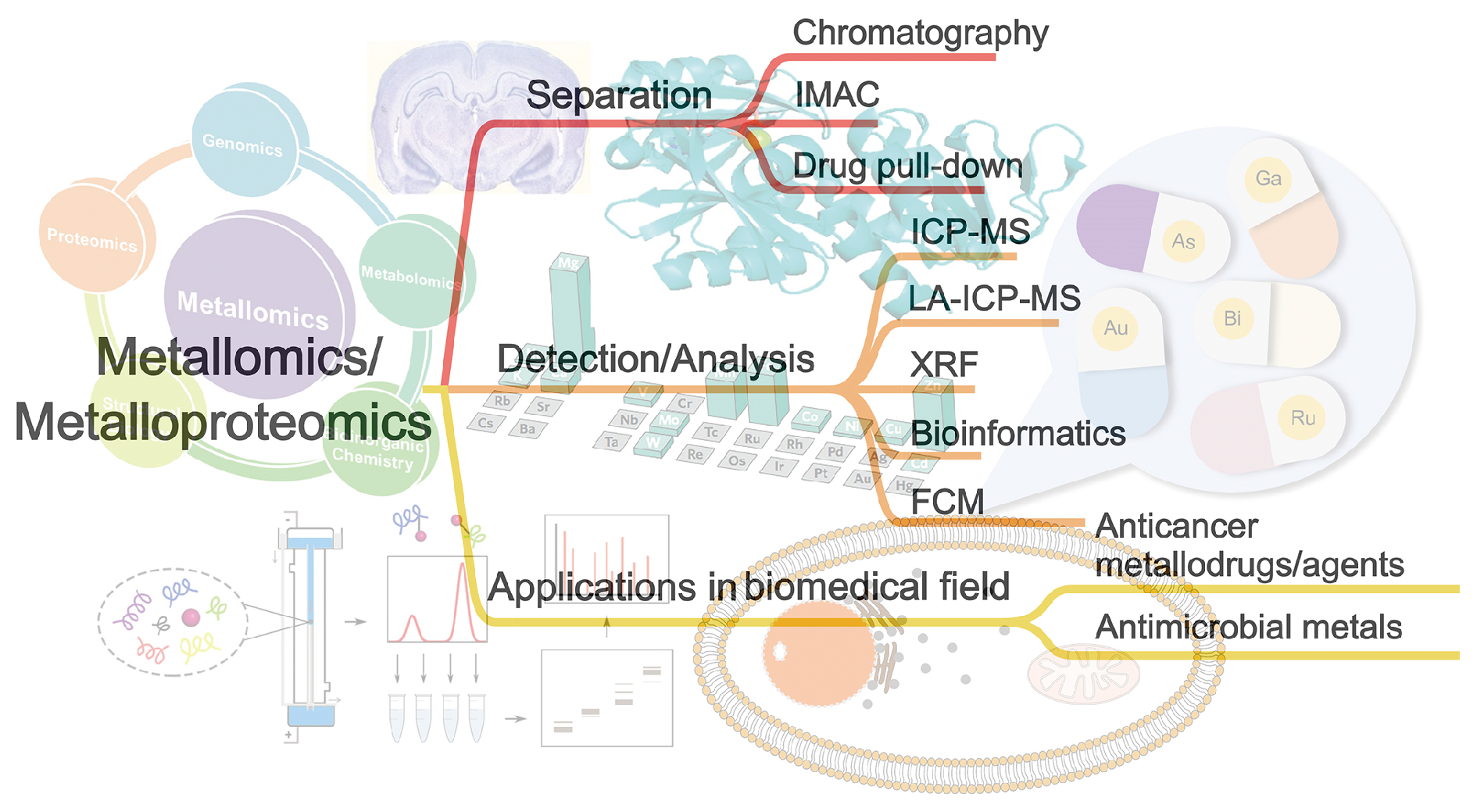
图1 金属蛋白质组学方法及应用。(A) 由ICP-MS和LC-MS/MS技术组成的传统整合分析方法;(B) 大肠杆菌胞浆中与Ag+相关的蛋白质图谱;(C)固定化金属亲和色谱法(IMAC);(D) 药物下拉实验的工作流程Fig.1 Illustration of metalloproteomics approaches and the applications. (A) Conventional integrated analytical methods consisted of ICP-MS and LC-MS/MS; (B) Map of Ag+-associated proteins in the E. coli cytosol; (C) The workflow of immobilized metal affinity chromatography (IMAC); (D) The workflow of drug pull-down assay |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
/
| 〈 |
|
〉 |