

Preparation and Extraction Application of Lithium Ion Selective Adsorption Materials
Received date: 2023-02-14
Revised date: 2023-07-01
Online published: 2023-08-07
Supported by
Science and Technology Major Projects of Xinjiang Autonomous Region(2022A03009)
National Natural Science Foundation of China(21975228)
In recent years, with the rapid advancement and large-scale application of lithium battery technology and electric vehicle, the market demand for lithium resource is growing sharply. However, due to insufficient mining degree and extraction technology, the total production capacity of ore lithium and brine lithium resources is far below the actual market demand. Extracting lithium from surface salt lake brine, deep brine and other liquid resources has the advantages of large resource potential and low extraction cost, which presents an important research direction in the lithium resource extraction field. Among available lithium extraction technologies, adsorption method is suitable for extracting lithium from low concentration and large volume liquid brine resources in China, and selective lithium ion adsorption materials are the core of adsorption method. In this review, we focus on the preparation and application of lithium ion selective adsorption materials for lithium extraction from brine. The preparation methods, adsorption properties and adsorption mechanisms of organic (crown ether), inorganic (aluminum-, manganese- and titanium-based adsorbents) and composite selective lithium adsorption materials are reviewed. This review provides a brief prospect for the design and development of new lithium adsorption materials, which may push forward the efficient extraction and utilization of lithium resources from salt lake brine.
1 Introduction
2 Crown ether adsorbents
2.1 Preparation of crown ether adsorbent
2.2 Selective lithium extraction performance
2.3 Selective lithium extraction mechanism
3 Alumina-based materials
3.1 Preparation of aluminum adsorbent
3.2 Selective lithium extraction mechanism of aluminum adsorbent
3.3 Selective lithium extraction performance of aluminum-based adsorbent
4 Lithium ion sieve adsorbent
4.1 Preparation of ion sieve adsorbent
4.2 Lithium ion insertion/extraction mechanism
4.3 Selective lithium extraction performanc of lithium ion sieve
4.4 Molded lithium ion sieve adsorbent
5 Other types of adsorbents
6 Conclusion and outlook

Key words: brine; lithium extract; adsorbent; ion sieve
Xinyi Chen , Kaisheng Xia , Qiang Gao , Zhen Yang , Yudie Li , Yi Meng , Liang Chen , Chenglin Liu . Preparation and Extraction Application of Lithium Ion Selective Adsorption Materials[J]. Progress in Chemistry, 2023 , 35(10) : 1519 -1533 . DOI: 10.7536/PC230214
表1 冠醚配体复合吸附材料的性能Table 1 Performance of crown ether ligand composite adsorption materials |
| Crown ether ligands | Matrixes | Specific surface area (m2/g) | pH | Adsorption capacity (mg/g) | Selectivity α(Li/Na) | Cycling stability | ref |
|---|---|---|---|---|---|---|---|
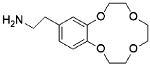 Aminoethylbenzo-12-crown-4 | Polymer nanosheets (PMBA-PMA) | / | 7 | 14.67 | / | 90% (Five cycles) | 30 |
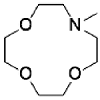 1-aza-12-crown-4 | mesoporous silica SBA-15 3-Aminopropyltriethoxy 4-ylsilane | 578 | 8 | 7.63 × 10-3 | / | / | 31 |
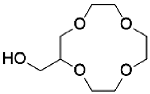 2- ( hydroxymethyl ) 12-crown-4 | graphene oxide chitosan polyvinyl alcohol | 101.5 | 7 | 168.50 | 2.51 | 88.31% (Five cycles) | 32 |
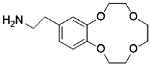 Aminoethylbenzo-12-crown-4 | Porous polymer substrate (PVBC) | / | 7 | 4.22 | 6.59 | 95.0% (Five cycles) | 35 |
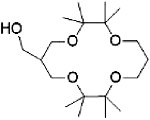 Octamethyl 14-crown-4 | methacrylate polymer | / | / | 3.05 | / | / | 36 |
1) 选择性系数α,也称分离因子,是表示某一单元分离操作或某一分离流程将两种物质分离的程度,其计算方法为 =Kd(Li+)/Kd(Me)。Me: K+、Na+、Ca2+、Mg2+。 2) Kd为吸附分配系数,是指一定温度达到反应平衡时,组分在固定相中的质量分数与流动相中的质量浓度之比,其大小反映离子在固液两相中的移动与分离能力。 |
表2 三种金属基吸附剂性能比较Table 2 Performance comparison of three metal-based adsorbent |
| Performance | Al-based | Mn-based | Ti-based |
|---|---|---|---|
| Li+ Adsorption Capacity | √ | √√ | √√√ |
| Li+ Selectivity | √ | √√√ | √√ |
| Technology Maturity | √√√ | √√ | √ |
| Stability and Regeneration Ability | √√√ | √ | √√ |
| Facile Operation Conditions | √√√ | √ | √√ |
| Environmental Safety | √√√ | √ | √√ |
| Low Preparation Cost | √√√ | √ | √√ |
表3 铝基吸附剂的合成方法与性能Table 3 Synthesis methods and properties of aluminum-based adsorbents |
| Adsorbent | Source | Method | Li+ adsorption capacity (mg/g) | pH | Selectivity (α) | Recovery rate | ref |
|---|---|---|---|---|---|---|---|
| LiAl-LDHs | AlCl3·6H2O NaOH Na2CO3 | Reaction coupling separation technology | / | / | / | 96.07% | 55 |
| MLDH (Fe3O4 doped LiAl-LDHs) | FeCl3·6H2O AlCl3·6H2O LiCl·H2O NaOH FeCl2·4H2O | Sectional chemical co-precipitation method | 5.83 | 7 | α(Li/Mg)= 362.68 | / | 56 |
| Al(OH)3 | AlCl3·6H2O NaOH brine | co-precipitation method | / | 7.5 | / | 76.4% | 57 |
| LiOH/Al(OH)3 | NaOH anhydrous aluminum chloride anhydrous lithium | single step co-precipitation | 15.06 | 6~7 | / | / | 58 |
| Li/Al-LDHs | Al(OH)3 LiOH·H2O | hydrothermal method | / | / | α(Li/Na)= 47.80 | 91% | 59 |
表4 不同类型钛基离子筛合成方法与性能Table 4 Synthesis methods and properties of different titanium-based ion sieves |
| Precursor | Source | Method | Li+ adsorption Capacity (mg/g) | pH | Selectivity (α) | Cycling stability | ref |
|---|---|---|---|---|---|---|---|
| Li4Ti5O12 | TTIP LiOH·H2O | solvothermal reaction | 35.5 | 13 | / | 92.5% (Five cycles) | 12 |
| Li4Ti5O12 | Ti3AlC2 LiOH | two-step hydrothermal method | 43.20 | 12.1 | α(Li/Mg) =269.00 | 93% (Twenty cycles) | 70 |
| Li4Ti5O12 | TiO2LiOH | Soft hydrothermal method | 39.43 | / | / | / | 81 |
| Li2TiO3 | C2H3LiO2·2H2O TiO2 | high-temperature calcination | 40.16 | 10 | α(Li/Mg) =5441.17 | 98% (Five cycles) | 87 |
| 3DM-Li4Ti5O12 | CH3COOLi C12H28O4Ti | hydrothermal method low temperature calcination method | 38.24 | / | α(Li/Mg) =30.00 | 80% (Six cycles) | 90 |
| Li2TiO3 | Ti(OBu)4 Li2CO3 | solid state reaction | 34.2 | 12 | α(Li/Na) =19.96 | 90.6% (Eight cycles) | 92 |
表5 不同类型锰基离子筛合成方法与性能Table 5 Synthesis methods and properties of different manganese ion sieves |
| Precursor | Source | Method | Li+ adsorption capacity | pH | Selectivity | Cycling stability | ref |
|---|---|---|---|---|---|---|---|
| 1-D Li4Mn5O12 | MnSO4LiNO3 | hydrothermal method low-temperature solid-phase reaction | 6.62 mmol/g | / | α(Li/Mg) =599.12 | / | 66 |
| LiMg0.56Mn1.50O4 | MnCl2·4H2O Mg(NO3)2·6H2O LiOH | soft chemical method | 37.4 mg/g | 12 | / | 95% (Four cycles) | 89 |
| LiMxMn2-xO4(M=Mg,Cu and Zn) | Li2CO3 CuO ZnO MgCO3MnO | high-temperature calcination | / | / | / | / | 88 |
| Li1.6(Mn0.7Al0.3)1.6O4 | MnO2LiCl AlCl3 | hydrothermal method | 32.32 mg/L | / | / | 95% (Five cycles) | 91 |
| Li1.33Mn1.67O4 | Li2CO3MnCO3 | solid-phase synthesis method | 10.00 mg/g | / | / | / | 95 |
| Li1.6Mn1.6O4 | KMnO4LiOH | hydrothermal method Solid high temperature sintering method | 41 mg/g | / | C =474.46 | 85.37% (Five cycles) | 97 |
表6 冠醚、铝基、锂离子筛型以及其他类型吸附剂的优缺点总结Table 6 Summary of advantages and disadvantages of crown ether, aluminum based, lithium-ion sieve type, and other types of adsorbents |
| Performance | Crown ether | Alumina-based adsorbent | Lithium ion-sieve | Others |
|---|---|---|---|---|
| Adsorption Capacity | ★★ | ★ | ★★★ | ★★ |
| Selectivity | ★★ | ★ | ★★★ | ★★ |
| Technology Maturity | ★ | ★★★ | ★★ | ★ |
| Stability and Regeneration | ★★ | ★★★ | ★ | ★★ |
| Cost | ★ | ★★★ | ★★ | ★ |
| [1] |
|
| [2] |
USGS. Mineral commodity summaries 2022, in: MineralCommodity Summaries, 2022.
|
| [3] |
(徐伟民, 肖坚, 丁冀荣. 老区建设, 2023, 3: 3.).
|
| [4] |
USGS. Reston, VA, 2023.
|
| [5] |
(陈立, 杨立, 刘韬, 高杰, 胡永碧, 刘友权. 石油与天然气化工, 2023, 52(2): 41.).
|
| [6] |
(代鸿章, 王登红, 刘善宝, 李鑫, 王成辉, 孙艳. 地质学报, 2023, 97(2): 583.).
|
| [7] |
(乜贞, 伍倩, 丁涛, 卜令忠, 王云生, 余疆江, 侯献华. 无机盐工业, 2022, 54(10): 1.).
|
| [8] |
(张宇轩. 中国经济周刊, 2023, 4: 42).
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(孙淑英, 张钦辉, 于建国. 无机材料学报, 2010, 25: 626.).
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
(奚干卿. 海南师范学院学报(自然科学版), 2001, 68.).
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
(梁苏卓成, 姬国勋, 孙新利, 李国东, 张仕通. 无机化学学报, 2021, 37(11): 2037.).
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
(郭敏, 刘忠, 李权, 吴志坚. 青海科技, 2019, 26: 16.).
|
| [50] |
|
| [51] |
|
| [52] |
(董茜, 李燕杰, 朴香兰, 朱慎林. 稀有金属, 2007, 3: 357.).
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
(程鹏高, 黄传峰, 甘善甜, 龚经款, 项军, 唐娜. 无机盐工业, 2021, 53(6): 140.).
|
| [59] |
|
| [60] |
(石西昌, 张志兵, 周定方, 周喜诚, 唐天罡, 尹世豪. 中南大学学报(自然科学版), 2013, 44(3): 892.).
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
(韩红静, 吴勇民, 曹永生, 韩婷婷, 汤卫平. 无机盐工业, 2022, 54(8): 59.).
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
(潘立玲, 朱建华, 李渝渝. 矿产综合利用, 2002, 2: 28.).
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
/
| 〈 |
|
〉 |