

Research Progress on Self-Healing Polyurethane and Its Applications in the Field of Flexible Sensors
Received date: 2023-05-30
Revised date: 2023-07-12
Online published: 2023-08-07
Supported by
The National Natural Science Foundation of China(52003278)
The National Natural Science Foundation of China(52211540393)
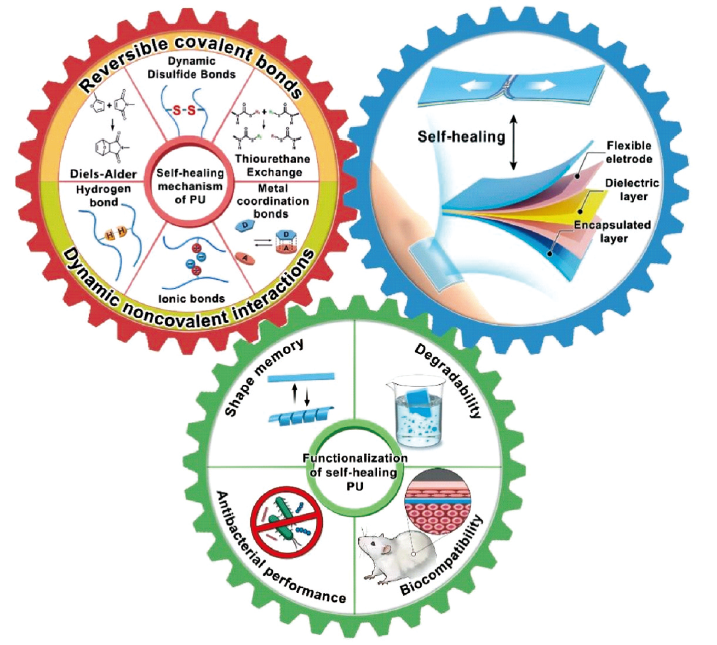
Polyurethane, a prevalent polymer, has garnered considerable attention owing to its exceptional overall performance within various applications. However, even minor damages can significantly curtail the service life of polyurethane. Consequently, a promising approach to address this challenge involves conferring self-healing properties upon polyurethane. Among the various healing mechanisms found in self-healing polyurethane, the intrinsic driving force stands out as the most common. This mechanism entails the spontaneous re-entanglement of polyurethane molecular chains through meticulous molecular structure design, obviating the necessity for external healing agents. Intrinsic driving force encompasses reversible covalent bonds (e.g., disulfide bonds, Diels-Alder reactions, and boronic ester bonds) as well as dynamic non-covalent interactions (e.g., hydrogen bonds, ionic bonds, metal coordination bonds, and host-guest interactions). The polyurethane main chain can possess a single intrinsic driving force or multiple intrinsic driving forces concurrently. Nevertheless, while self-healing polyurethane alone presents advantages in terms of extending service life and reducing maintenance costs through damage repair, it still falls short of meeting the usage requirements in certain specialized applications. To further enable the versatile application of self-healing polyurethane while preserving its self-healing properties, the incorporation of new functional groups becomes an enticing prospect. These functional groups can bestow specific properties upon polyurethane, such as shape memory, degradability, antibacterial properties and biocompatibility, thereby achieving functional integration within self-healing polyurethane. Importantly, these functionalized self-healing polyurethanes possess the potential to supplant traditional materials as dielectric materials, substrate materials, or encapsulation materials in the realm of flexible sensors. Consequently, they contribute to enhancing the reliability and durability of flexible sensors. Therefore, this article primarily focuses on elucidating the self-healing mechanism of self-healing polyurethane. Subsequently, it delves into the integration of functionality within self-healing polyurethane and its application within the field of flexible sensors. Lastly, based on these insights, the paper provides a glimpse into the future prospects for the development of self-healing polyurethane.
1 Introduction
2 Self-healing mechanism of polyurethane(PU)
2.1 Reversible covalent bonds
2.2 Dynamic noncovalent interactions
2.3 Combination of covalent bonds and noncovalent interactions
3 Functionalization of self-healing polyurethane
3.1 Shape memory
3.2 Degradability
3.3 Antibacterial performance
3.4 Biocompatibility
4 Application of self-healing PU in flexible sensors
4.1 Self-healing PU based dielectric layer
4.2 Self-healing PU based flexible electrode
4.3 Self-healing PU based encapsulated layer
5 Conclusion and outlook
Chao Chen , Guyue Wang , Ying Tian , Zhengyang Kong , Fenglong Li , Jin Zhu , Wu Bin Ying . Research Progress on Self-Healing Polyurethane and Its Applications in the Field of Flexible Sensors[J]. Progress in Chemistry, 2023 , 35(9) : 1275 -1293 . DOI: 10.7536/PC230530
图2 (a) Diels-Alder反应;(b) Diels-Alder反应的自愈合机理;(c) 含有Diels-Alder反应的聚氨酯和 (d) 不含Diels-Alder反应的聚氨酯在一定温度下的自愈合图片[20]Fig.2 (a) Diels-Alder interaction; (b) Self-healing mechanism of Diels-Alder interaction; Self-healing pictures of polyurethane with Diels-Alder reaction(c) and polyurethane without Diels-Alder reaction (d) at a certain temperature[20]. Copyright 2019, American Chemical Society |
图3 (a) BS-PU的化学结构;(b) 拉长的PU膜示意图,裂缝可以在动态二硫键的驱动下自我修复(右);(c)缺口和自愈的BS-PU-3薄膜的光学显微镜图像;(d) 自愈合后的BS-PU的560 g的举重测试[29]Fig.3 (a) Chemical structure of BS-PU; (b) Schematic of an elongated PU film, and the crack could be self-healed driven by dynamic disulfide bonds (right); (c) Optical microscope images of the notched and self-healed BS-PU-3 film; (d) Weight lifting test demonstrating the self-healing capability of BS-PU with a load of 560 g[29]. Copyright 2020, American Chemical Society |
图4 (a) 自愈合聚氨酯 (CBPU) 中的动态键:硫代氨基甲酸乙酯交换[35];(b) 含有硫代氨基甲酸酯键的聚氨酯的自愈合图像[35];(c) 可见光照射下的二硒化合作用[36];(d) 含有二硒键的自愈合聚氨酯在压力下的愈合行为:光照24 h后裂纹消失[36]Fig.4 (a) Dynamic bonds contained in self-healing polyurethanes (CBPU): thiourethane exchange (b) Optical self-healing microscope images of polyurethanes containing thiourethane bonds[35]; (c) Diselenide metathesis under visible light irradiation[36]; (d) Healing behavior under pressure; the crack disappeared after 24 h light irradiation[36]. Copyright 2018, American Chemical Society |
图5 (a) 含有非平面环和 (b) 含有苯环的多重氢键聚氨酯的结构式;(c) 自愈合聚氨酯在一定温度下划痕消失的显微图[46]Fig.5 Structure of polyurethanes with multiple hydrogen bonds featuring (a) non-planar rings and (b) benzene rings;(c) Microscope images of self-healing polyurethane scratch disappearance at a certain temperature[46]. Copyright 2021, Willey |
图7 (a) 金属配位键的自愈合机理和 (b) 含有金属配位键的自愈合聚氨酯在一定温度下的划痕消失图[62];(c) Donor-Acceptor相互作用示意图和含有Donor-Acceptor相互作用的自愈合聚氨酯在一定温度下自愈合的偏光显微图[67]Fig.7 (a) Self-healing mechanism of metal ligand bonds; (b) Digital photos and optical microscope photos of the cutting-healing-stretching procedure of self-healing polyurethanes containing metal ligand bonds[62]; (c) Schematic illustration of the breakup and restore of Donor-Acceptor self-assembly and (d) micrographs of self-healing polyurethane containing Donor-Acceptor at certain temperatures[67]. Copyright 2021, Willey |
图10 (a) 自愈合聚氨酯 (CBPU) 中含有的动态键:硫代氨基甲酸酯键;(b) 自愈合聚氨酯的自愈合偏光显微图;(c) 自愈合聚氨酯的抗菌测试[35]Fig.10 (a) Dynamic bonds contained in self-healing polyurethanes (CBPU): thiourethane exchange; (b) Optical self-healing microscope images of polyurethanes containing thiourethane bonds; (c) Antibacterial testing of self-healing polyurethane[35]. Copyright 2021, Elsevier |
图11 (a) 具有生物相容性的自愈合聚氨酯的结构示意图;(b) 具有生物相容性的自愈合聚氨酯的自愈合演示;(c) 细胞在具有生物相容性的自愈合聚氨酯上生长的荧光染色图[113]Fig.11 (a) Scheme of a self-healing polyurethane with biocompatibility; (b) Demonstration of self-healing with biocompatible self-healing polyurethane; (c) Fluorescent staining of cells grown on biocompatible self-healing polyurethane[113]. Copyright 2022, American Chemical Society |
图14 (a) 以自愈合聚氨酯为封装层制备传感器的示意图;(b) 封装后传感器的传感性能;(c) 聚氨酯封装层的自愈合性能示意图[138]Fig.14 (a) Illustration of sensor fabrication using self-healing polyurethane as an encapsulation layer; (b) Sensing performance of the encapsulated sensor; (c) Illustration of self-healing properties of the polyurethane encapsulation layer[138]. Copyright 2020, American Chemical Society |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
(张广伟, 张来斌, 樊建春, 孙秉才, 齐立娟, 赵坤鹏, 张仁庆. 无损检测, 2013, 35(1): 56.
(
|
| [132] |
(周国鹏. 压电与声光, 2010, 32(4):534.).
|
| [133] |
任越, 张钰民, 钟国舜, 宋言明, 孟凡勇. 激光与红外, 2020, 50(5): 598.
(
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
/
| 〈 |
|
〉 |