

Oxygen Permeability of Polymer Hydrogel Materials
Received date: 2023-02-14
Revised date: 2023-06-07
Online published: 2023-08-06
Supported by
The National Natural Science Foundation of China(22275035)
Jiangsu Key Research and Development Program(BE 2022025-2)
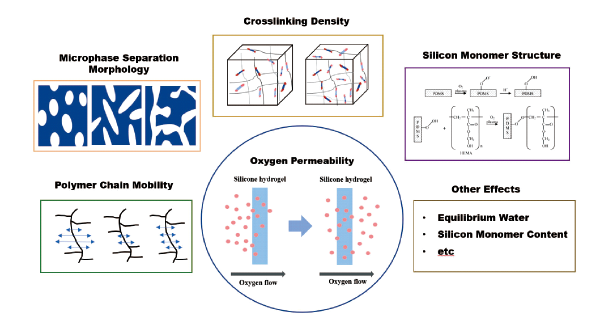
In recent years, hydrogels have been widely used in biomedical fields such as contact lenses and medical dressings. In these fields, oxygen permeability is a key index to evaluate the application performance of hydrogel materials. In this paper, the application of traditional hydrogels and silicone hydrogels in the field of corneal contact lenses and medical dressings is sketched. The research progress of traditional hydrogels and silicone hydrogels in structural design and oxygen permeability mechanism is summarized, and various factors affecting the oxygen permeability of silicone hydrogels are analyzed emphatically. It is hoped that the relationship between hydrogel microstructure and oxygen permeability can be further understood by summarizing and sorting out the recent related research work, so as to provide help for the regulation of material properties and the design of materials to meet the requirements.
1 Introduction
2 Progress in oxygen permeability of traditional hydrogels
2.1 Research progress of traditional hydrogels as contact lenses
2.2 Oxygen permeability mechanism of hydrogel materials
3 Progress in oxygen permeability of silicone hydrogels
3.1 Research progress of silicone hydrogels as contact lenses
3.2 Oxygen permeability mechanism of silicone hydrogel materials
4 Conclusion and outlook
Shiping Jin , Ying Sun , Xueqin Zhang . Oxygen Permeability of Polymer Hydrogel Materials[J]. Progress in Chemistry, 2023 , 35(9) : 1304 -1312 . DOI: 10.7536/PC230213
表1 传统水凝胶和硅水凝胶研究工作总结Table 1 Summary of the work of traditional hydrogels and silicone hydrogels |
| Component | Preparation method | Oxygen permeability | Water content | ref | |
|---|---|---|---|---|---|
| Traditional hydrogels | HEMA、MPC | Copolymerization | 33 barrer | 58% | 17 |
| HEMA、MA、MMA | Ontology aggregation | 35 barrer | 45% | 23 | |
| NVP、HEMA | Copolymerization | 35 barrer | 84.5% | 19 | |
| Silicone hydrogels | KH570、HEMA、NVP | Ontology aggregation | 35 barrer | / | 43 |
| SIGMA、HEMA、NVP | Hydrate after copolymerization | 34.5 barrer | / | 44 | |
| PDMS、PEGMA、HEMA | Copolymerization | 92 barrer | / | 48 | |
| PDMS、PTMO、HMDI | Copolymerization | 100 barrer | / | 49 | |
| PEG、PDMS | Copolymerization | 110 barrer | / | 18 | |
| PDMS、PEG、 Fluorohydrocarbon groups | Copolymerization | 196 barrer | / | 50 | |
| TEOS、PDMS、HEMA、NVP | Photopolymerization | 71 barrer | 73% | 52 |
| [1] |
(李倩. 河北大学博士论文, 2011.).
|
| [2] |
(魏清渤, 白志洋, 江源. 应用化工, 2013, 42(1): 62.).
|
| [3] |
|
| [4] |
(汤琦. 东华大学硕士论文, 2010.).
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
(王延龙. 青岛科技大学硕士论文, 2014.).
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
(黎新明, 崔英德, 蔡立彬. 高分子材料科学与工程, 2004, ( 02): 191.).
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
(王泽巩. 深圳大学硕士论文, 2018.).
|
| [25] |
(杨漪, 邓军, 程方明, 赵大龙, 唐凯. 功能材料, 2014, 45(S2): 129.).
|
| [26] |
(艾媛媛. 陕西师范大学硕士论文, 2013.).
|
| [27] |
(杨菲, 刘波, 罗仲宽, 周莉, 梁汉秋, 李明. 化学研究, 2015, 26(01): 90.).
|
| [28] |
(任秀彬, 杨来侠, 张亚刚. 煤炭与化工, 2015, 38(12): 22.).
|
| [29] |
|
| [30] |
(黎新明. 西北工业大学博士论文, 2003.).
|
| [31] |
|
| [32] |
(谭帼馨, 崔英德, 易国斌, 周家华. 化工学报, 2005, (10): 2019.).
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
(蔡立彬. 西北工业大学博士论文, 2006.).
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
方江邻, 余学海. 高分子材料科学与工程, 1998, 14(3): 15.).
|
| [50] |
岩田淳一, 保木恒夫, 井川诚一郎, 阿瑟·巴克. CN 102323629B, 2015.
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
/
| 〈 |
|
〉 |