

Selective Oxidative Lactonization of 1,6-Hexanediol into ε-Caprolactone
Received date: 2022-12-15
Revised date: 2023-05-08
Online published: 2023-07-18
Supported by
National Natural Science Foundation of China(22172010)
Fundamental Research Funds for the Central Universities(DUT2021TD103)
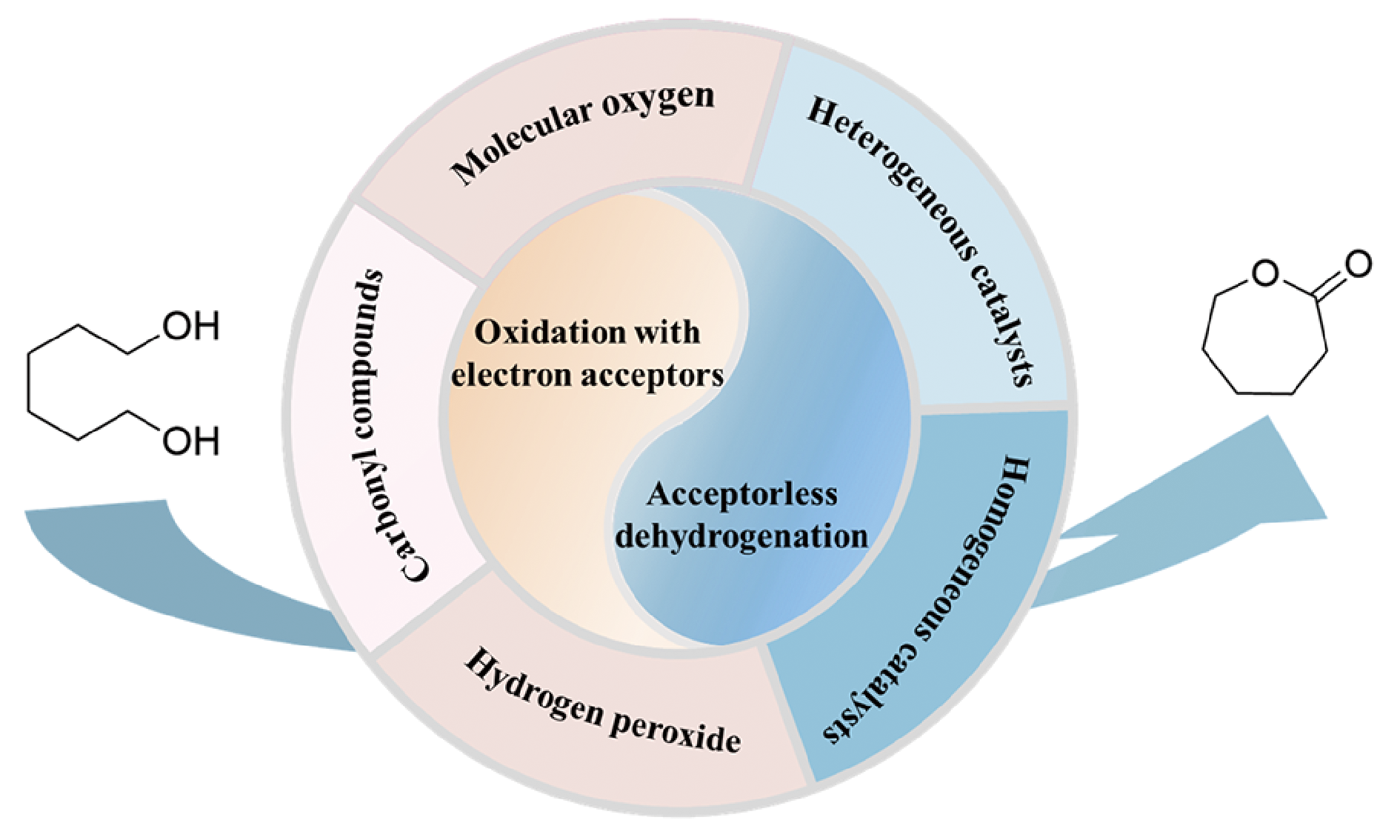
ε-Caprolactone is a key monomer for the synthesis of poly(ε-caprolactone) (PCL) with good biocompatibility and biodegradability, and relevant polymer materials could be applied in pharmaceutical, medicinal, and packaging applications. Green and economic synthesis of ε-caprolactone is vital to popularize such eco-friendly polymers, and selective oxidative lactonization of 1,6-hexanediol into ε-caprolactone remains to be developed. In this review, different routes for the synthesis of ε-caprolactone such as Baeyer-Villiger oxidation of cyclohexanone and oxidative lactonization of 1,6-hexanediol are comparatively analyzed. According to whether electron acceptors (oxidants) are added to the reaction systems, the related advances of oxidative lactonization of 1,6-hexanediol are summarized, and the advantages and disadvantages of the corresponding reaction systems and catalysts are reviewed. The development trend of oxidative lactonization of 1,6-hexanediol into ε-caprolactone is also proposed.
1 Introduction
2 Catalytic oxidation processes
2.1 Carbonyl compounds act as electron acceptors
2.2 Molecular oxygen acts as the electron acceptor
2.3 H2O2acts as the oxidant
3 Catalytic dehydrogenation
3.1 Homogeneous catalysts
3.2 Heterogeneous catalysts
4 Conclusion and outlook
Xiaoyu Shen , Zhongtian Du , Bairui Guo , Zhongxu Guo , Changhai Liang . Selective Oxidative Lactonization of 1,6-Hexanediol into ε-Caprolactone[J]. Progress in Chemistry, 2023 , 35(8) : 1191 -1198 . DOI: 10.7536/PC221209
表1 1,6-己二醇氧化内酯化为ε-己内酯反应的原子经济性、理论副产物、可能安全隐患的对比1)Table 1 Comparison of atom economy, theoretical by-products, and possible safety hazards in oxidative lactonization of 1,6-hexanediol into ε-caprolactone1) |
| Electron acceptors | Atom utilization [%] | Theoretical by-product | Possible safety hazards |
|---|---|---|---|
| Methyl isobutyl ketone2) | 35.8% | 4-Methyl-2-pentanol | Volatile solvent |
| O2 | 87.7% | H2O | O2 and organic mixture |
| H2O2 | 44.2% | H2O | Storage and transport of H2O2 |
| Electron acceptor-free (Dehydrogenation reaction) | 96.6% | H2 | Explosion limit of H2 |
Table note:1) Catalysts and additives are not discussed in this table;2) Methyl isobutyl ketone (MIBK) is used as the example of carbonyl compounds. |
| [1] |
(张涵, 孙志强, 李帅, 庞烜, 陈学思. 高分子材料科学与工程, 2021, 37(1): 218.).
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
国家发展改革委生态环境部关于进一步加强塑料污染治理的意见, 发改环资〔2020〕80号, 2020.
|
| [7] |
|
| [8] |
(严生虎, 韩玲玲, 沈卫, 沈介发, 刘建武, 张跃. 化工进展, 2014, 33(11): 3061.).
|
| [9] |
(袁浩然, 汪玲瑶, 杜仁峰, 姚加, 李浩然. 中国科学: 2020, 50(2): 245.).
|
| [10] |
(鲁华, 高伟. 精细与专用化学品, 2013, 21(7): 9.).
|
| [11] |
(丁璟, 赵俊琦, 程时标, 慕旭宏, 宗保宁. 化工进展, 2015, 34( 12): 4209.).
|
| [12] |
(高芳芳, 陈静, 黄志威, 夏春谷. 分子催化, 2018, 32(3): 276.).
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
(闫捷, 赵立红, 宋灿, 蒋元力, 魏灵朝. 化工进展, 2017, 36(4): 1424.).
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
(刘燕, 周茜, 郑长义, 王玉忠. 现代化工, 2007, (10):41.).
|
| [53] |
(吴彦彬, 宋国全, 闫广学, 吴正岭. 精细与专用化学品, 2015, 23(1): 37.).
|
| [54] |
|
| [55] |
|
/
| 〈 |
|
〉 |