

Structure Design and Tailoring Strategy of Polymeric Materials for Fabrication of Nanofiltration Membranes via Phase Inversion
Received date: 2022-12-28
Revised date: 2023-06-14
Online published: 2023-07-18
Supported by
National Natural Science Foundation of China(21978215)
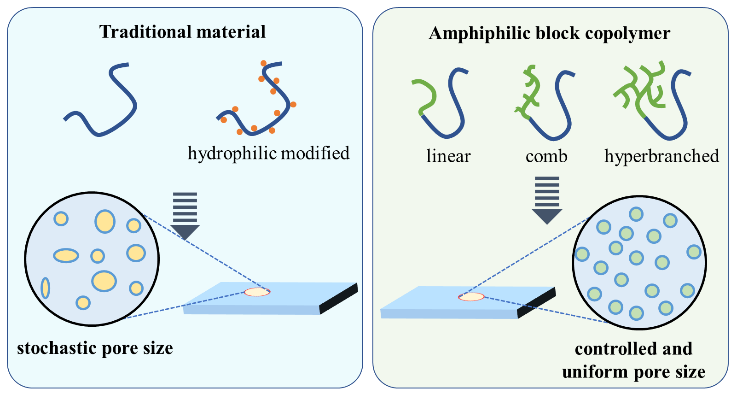
The non-solvent induced phase separation (NIPS) method has significant advantages including easy processing and tailorable membrane structure in the preparation of nanofiltration membranes with high-flux and selectivity. Increasing attention has been drawn from the membrane field to further improve the precise separation and permeability of the membrane. In this review, the effects of the thermodynamics and kinetics on the membrane structure and properties during the NIPS process are systematically described, and the research progress is summarized to illustrate how the polymeric membrane materials including polysulfone and polyethersulfone affect the membrane structure and separation performance. Furthermore, the characteristics of amphiphilic block copolymer materials and their outstanding advantages in the fabrication of high-flux nanofiltration membranes are comprehensively reviewed. Finally, the potential research focus is proposed to inspire the membrane community to develop high-performance nanofiltration membranes via NIPS in the future.
1 Introduction
2 Research progress of nanofiltration membrane prepared by phase inversion
2.1 Formation mechanism of nanofiltration membrane prepared by phase inversion
2.2 Materials for preparation of nanofiltration membrane by phase inversion
2.3 Optimization of nanofiltration membrane structure and separation performance
3 Amphiphilic block copolymers and the fabricated nanofiltration membranes
3.1 Amphiphilic block copolymer membrane materials and their characteristics
3.2 Research progress of block copolymer nanofiltration membrane
4 Conclusion and outlook
Tao Liu , Junping Miao , Longlong Wang , Yunxia Hu . Structure Design and Tailoring Strategy of Polymeric Materials for Fabrication of Nanofiltration Membranes via Phase Inversion[J]. Progress in Chemistry, 2023 , 35(8) : 1199 -1213 . DOI: 10.7536/PC221215
图2 不同铸膜液成分发生分相后所形成的膜结构:(Ⅰ,Ⅵ)致密结构;(Ⅱ)海绵孔结构;(Ⅲ)双连续结构;(Ⅳ)粒状结构(黄色代表聚合物富相,绿色代表聚合物贫相)[27]Fig.2 The membrane structure formed by phase inversion of the different casting solution components: (Ⅰ,Ⅵ) Dense structure; (Ⅱ) Sponge structure; (Ⅲ) Bi-continuous or lacy structure; (IV) Nodules (yellow represents polymer rich phase and green represents polymer lean phase)[27]. Copyright 1990, Elsevier |
图7 (a)不同热处理温度下所成CA膜的外皮层形貌(CA-1# 未处理,CA-2# 60℃处理,CA-3# 90℃处理),(b)CA-3#膜断面不同区域的形貌[72]Fig.7 (a) Outer skin layer morphology of the CA membranes at different heat-treatment temperatures: CA-#1(untreated), CA-#2 (60 ℃) and CA-#3 (90 ℃), (b) cross-section, inner surface and outer surface morphology of the CA-#3 membrane[72]. Copyright 2010, Elsevier |
表1 相转化法所制纳滤膜的材质及分离性能参数指标Table 1 Main information (material, MWCO/pore size, water permeance and rejection) of NF membranes prepared through a phase inversion process |
| Casting material | MWCO/ pore size | Permeance (L/(m2·h·bar) | Solute rejection | ref |
|---|---|---|---|---|
| Polyamic acid | 800 Da | 4 | R(Na2SO4) = 94% | 93 |
| Sulfonated poly(phthalazinone biphenyl ether sulfone) | - | 7 | R(Na2SO4) = 84% | 94 |
| Polyetherimide | - | 7 | R(Na2SO4) = 76% | 95 |
| Sulfonated nitro-polyphenylsulfone | - | 8 | R(Na2SO4) = 65% | 57 |
| Polyacrylonitrile-co-methylacrylate | - | 15 | R(Na2SO4) = 30% | 96 |
| Polyacrylonitrile-graft-poly(ethylene oxide) | 1 nm | 9 | R(Congo Red) = 100% R(Ethyl Orange) = 81% | 97 |
| Poly(trifluoroethyl methacrylate)-r- sulfobetaine methacrylate | 1 nm | 8 | R(Brilliant Blue R) = 100% | 98 |
| Carboxylated cardo poly(arylene ether ketone)s | 4 nm | 30 | R(Congo red) = 100% | 59 |
| Poly(m-phenylene isophthalamide) | - | 20 | R(Alizarine red) = 98% | 84 |
图10 (a)AB二嵌段共聚物微相分离所成形态,(b)由自洽场理论预测的AB嵌段共聚物的理论相图,(c)聚异戊二烯-聚苯乙烯嵌段共聚物的实验相图[130]Fig.10 (a) Equilibrium morphologies of AB diblock copolymers in bulk, (b) theoretical phase diagram of AB diblocks predicted by the self-consistent mean-field theory; (c) experimental phase diagram of polyisoprene-block-polystyrene copolymers[130]. Copyright 2012, Royal Society of Chemistry |
图13 通过Au3+和P4VP链段的络合作用,制备孔径可调的PS-b-P4VP嵌段共聚物膜:(a)Au3+和单一胶束的络合作用,(b)经Au3+络合还原后膜孔径的变化[152]Fig.13 The preparation of a PS-b-P4VP membrane with controlled pore sizes through electroless gold deposition: (a) gold decoration on a single micelle of PS-b-P4VP and (b) pore evolution of the PS-b-P4VP membrane with gold deposition[152]. Copyright 2014, Wiley |
图14 PS-b-P(HTMB-r-I)嵌段共聚物原始膜I0(a)及磺化膜SM(b)的示意图、聚合物化学结构及SEM图片,(c)原始膜I0和磺化膜SM水渗透性能的比较,(d)原始膜I0和磺化膜SM对酸性橙Ⅱ染料和活性绿19染料的分离性能[154]Fig.14 Schematic representation, chemical structure and SEM images of (a) the PS-b-P(HTMB-r-I) membrane I0, (b) the sulfonated membrane SM. (c) Comparison of water permeance of the pristine membrane I0 and the sulfonated membrane SM under trans-membrane pressure of 1 bar. (d) The separation behavior of small organic molecules (i.e. orange II and reactive green 19) using the membranes I0 and SM[154]. Copyright 2020, Royal Society of Chemistry |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
/
| 〈 |
|
〉 |