

Interactions Between Humic Acid and Co-Existing Substances in Aquatic Environments
Received date: 2022-12-10
Revised date: 2023-04-13
Online published: 2023-06-15
Supported by
National Key R&D Program of China(2019YFC0408801)
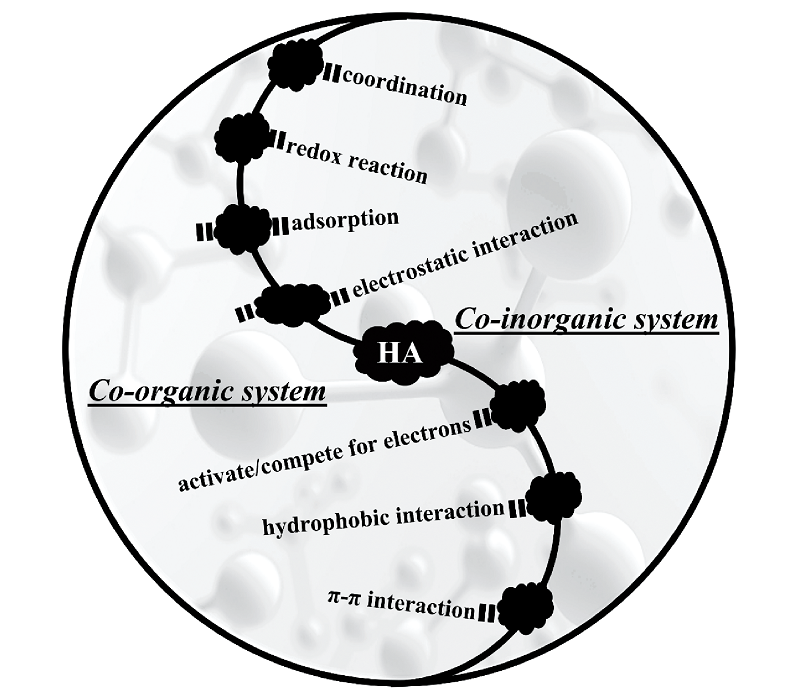
Humic acid (HA) has attracted significant attention in the field of environmental remediation due to its occurrence characteristics and unique chemical reactivity. It is worth noting that in co-existing reaction systems, HA inevitably interacts with co-existing substances, making the reaction system complex and leading to unexpected results. Therefore, studying the interaction between HA and co-existing substances is of great significance for a correct understanding of the complexity of environmental water pollution and the development of new environmental functional materials with cooperative treatment of co-existing substances. This article reviews the synergistic/antagonistic removal effects of target pollutants in co-existing pollution systems involving HA, including inorganic co-existing pollutant systems, organic co-existing pollutant systems, and microbial co-existing systems. Based on the structural characteristics and physicochemical properties of HA itself, the interaction mechanisms between HA and co-existing pollutants are systematically analyzed. These mechanisms mainly involve coordination, electrostatic interactions, adsorption, hydrophobic interactions, π-π interactions, and oxidation-reduction reaction (REDOX). Finally, the challenges and future research directions for the removal of target pollutants by HA in co-existing pollution systems are discussed.
1 Introduction
2 Removal of target contaminant in different co-existing systems
2.1 HA with co-inorganic contaminant system
2.2 HA with co-organic contaminant system
2.3 HA with co-microbial system
2.4 Quantitative comparison of the removal effectiveness in HA co-existing contaminant systems
3 Interaction mechanisms between HA and co-existing contaminant
3.1 HA with co-inorganic contaminant system
3.2 HA with co-organic contaminant system
3.3 HA with co-microbial system
3.4 Characteristics of interaction mechanism in HA co-existing contaminant systems
4 Conclusion and Outlook
Chundi Zhou , Minghao Sui . Interactions Between Humic Acid and Co-Existing Substances in Aquatic Environments[J]. Progress in Chemistry, 2023 , 35(7) : 1018 -1029 . DOI: 10.7536/PC221203
图2 HA参与共存体系下污染物的去除效果Fig.2 The removal synergistic or antagonistic effect of contaminants with HA in co-existing system |
表1 重金属氧化还原电位Table 1 Standard redox potential of heavy metal ions |
| Cr (Ⅵ) | Fe (Ⅲ) | Pb (Ⅱ) | Cd (Ⅱ) | Mg (Ⅱ) | Na (Ⅰ) | Ca (Ⅱ) | K (Ⅰ) | |
|---|---|---|---|---|---|---|---|---|
| E0 (Ⅴ) | 1.33 | 0.77 | -0.13 | -0.40 | -2.37 | -2.71 | -2.87 | -2.93 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
/
| 〈 |
|
〉 |