

Mechanism of Phase Transition on Zero-Valent Aluminum Surface and Its Effect on Pollutant Removal
Received date: 2022-11-24
Revised date: 2023-04-05
Online published: 2023-06-12
Supported by
Natural Science Foundation of Shandong Province(ZR2020MB093)
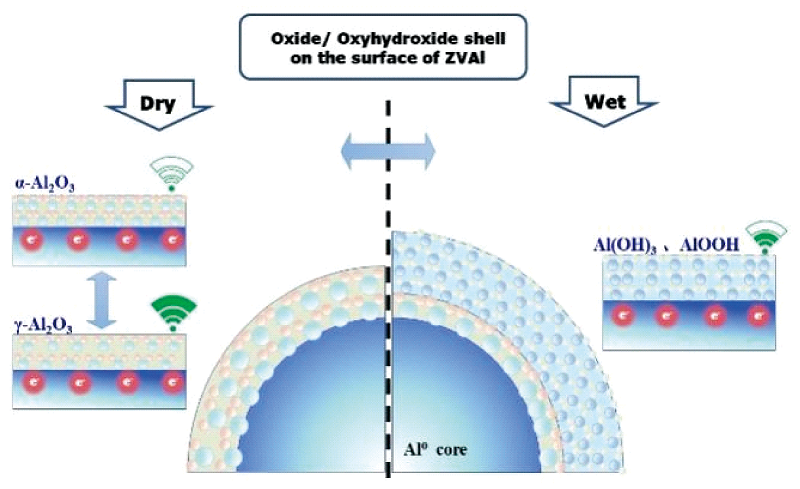
Zero-valent aluminum (ZVAl) is susceptibly oxidized in both gas and liquid media, which makes the element Al, as an “electron reservoir”, surrounded by an oxide/oxyhydroxide shell. Typically, this shell is made up of Al2O3, AlOOH, Al(OH)3 and other phases with varying structures. Furthermore, as the environment changes, the shell’s phases may transform into each other, and even the transition between different crystalline forms of the same phase may take place, finally leading to changes in the general properties of ZVAl. It is believed that the treatment of ZVAl in a variety of fields can be regarded as different regulations of its surface composition. Although it has been demonstrated that ZVAl can efficiently degrade pollutants due to its strong reducing ability, current research only focuses on the removal of the inherent oxides/oxyhydroxides on the surface of ZVAl, ignoring the transition and connection between the various phases. As a result, it is challenging to systematically clarify the impact of surface phase transformation on the reduction performance of ZVAl in the process of pollutant degradation. To provide a theoretical foundation for the investigation of the interfacial reaction processes and mechanisms between ZVAl and pollutants as well as the directional regulation of ZVAl, it is necessary to have a thorough understanding of the structure and properties of the various phases that make up the ZVAl surface, particularly the transition processes between different phases. Hence, in this review, for the first time, the reaction mechanism of the surface phase transition of ZVAl-based materials is summarized and prospectively discussed from the perspective of the type, structure, and nature of ZVAl surface phases as well as the reaction mechanism of the phase transition.
1 Introduction
2 Structure and properties of oxidized ZVAl in medias
2.1 Structure and properties of surface phases in gas media
2.2 Structure and properties of surface phases in liquid media
3 Phase transition of oxide/oxyhydroxide shells of ZVAl
3.1 To formγ-Al2O3
3.2 To formα-Al2O3
4 The influence mechanism of phase transition
4.1 Transition mechanisms in gas media
4.2 Transition mechanisms in liquid media
5 Conclusions and outlook
Shiying Yang , Zhen Yang . Mechanism of Phase Transition on Zero-Valent Aluminum Surface and Its Effect on Pollutant Removal[J]. Progress in Chemistry, 2023 , 35(7) : 1030 -1039 . DOI: 10.7536/PC221123
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
(杨世迎, 张艺萱, 郑迪, 辛佳. 化学进展, 2017, (08):879.).
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
(刘静, 顾天航, 王伟, 刘爱荣, 张伟贤. 化学学报, 2019, 77(02):121.).
|
| [16] |
(刘静, 刘爱荣, 张伟贤. 环境化学, 2014, 33(04):576.).
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
(甘丹丹. 中国石油大学(北京)硕士论文, 2016.).
|
| [34] |
|
| [35] |
(王正洲, 文广玉, 赵立功, 赵培丽, 蒋仁辉, 庄园林, 孟琪, 贾双凤, 郑赫, 王建波. 电子显微学报, 2020, 39(05):487.).
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
HÉrisson de Beauvoir T,
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
(王志平, 孙宇博, 丁坤英, 刘佳, 蔡珣. 焊接学报, 2008, 29(12):74.).
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
(袁超, 李磊, 孙应龙, 王邦达, 徐辉, 王毅. 环境科学研究, 2016, 29(07):1067.).
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
(李勐卓, 程继夏, 顾军农, 李玉仙, 邹放, 晏明全. 环境工程学报, 2021, 15(02):580.).
|
| [93] |
|
| [94] |
|
/
| 〈 |
|
〉 |