

Strong Metal-Support Interactions of Metal/Meatal Oxide Catalysts
Revised date: 2023-05-17
Online published: 2023-05-25
Supported by
The National Natural Science Foundation of China(22102007)
The National Natural Science Foundation of China(21991150)
The National Natural Science Foundation of China(22172150)
The National Natural Science Foundation of China(21821004)
The National Natural Science Foundation of China(22072090)
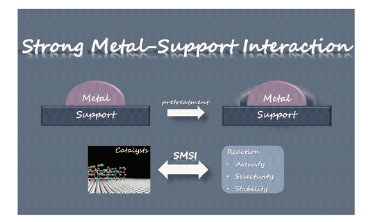
Catalysis plays an important role in the modern chemical industry, and developing catalyst with high efficiency is one of the important targets in catalysis research. Due to the outstanding activity of those catalysts with strong metal-support interactions (SMSI), SMSI has become an important scientific topic in catalysis research. The SMSI phenomenon involves the encapsulation of the metal nanoparticles (NPs) by support, resulting in the improved stabilization of NPs, and the different catalytic performances due to the new interaction between NPs and the support. Currently, a great number of catalysts with SMSI have been designed and partially applied, also there are considerable literatures focusing on SMSI of supported catalysts, especially those using metal oxide for support. However due to the complexity, the nature of SMSI and the catalytic mechanism of SMSI deserve further study, and the argument about the driving force of SMSI formation still exists. This review summarizes the recent progress, effect, and the regulation of SMSI, hopefully providing the understanding of SMSI from the perspective of condensed matter chemistry, and a new strategy of catalyst design.
1 Introduction
2 Research progress in SMSI
2.1 Research history of SMSI
2.2 New types of SMSI
3 Influence of SMSI on catalytic performance
3.1 Activity and stability enhancement
3.2 Selectivity tuning
4 Modulating of SMSI
4.1 Pre-treatment conditions
4.2 Supports
4.3 Metal nanoparticles
5 Conclusion and outlook
Xuetao Qin , Ziqiao Zhou , Ding Ma . Strong Metal-Support Interactions of Metal/Meatal Oxide Catalysts[J]. Progress in Chemistry, 2023 , 35(6) : 928 -939 . DOI: 10.7536/PC221226
图3 (a)Pt1/Co3O4、Pt1/CeO2、Pt1/ZrO2、Pt1/石墨烯、Pt箔以及PtO2的样品在Pt L3边的XANES谱;(b)傅里叶变换EXAFS谱;(c)Pt1/Co3O4、Pt1/CeO2、Pt1/ZrO2三个样品的漫反射红外CO吸收谱;(d)Pt1/Co3O4、Pt1/CeO2、Pt1/ZrO2、Pt1/石墨烯和PtO2样品在Pt 4f区域内的XPS谱图[42]Fig.3 (a) XANES spectra of Pt1/Co3O4, Pt1/CeO2, Pt1/ZrO2, and Pt1/graphene SACs as well as the Pt foil and PtO2 reference at the Pt L3-edge; (b) the corresponding K2-weighted Fourier transform spectra; (c) DRIFTS of CO chemisorption on Pt1/Co3O4, Pt1/CeO2, and Pt1/ZrO2 at the saturation coverage; (d) XPS spectra of Pt1/Co3O4, Pt1/CeO2, Pt1/ZrO2, Pt1/graphene, and PtO2 in the Pt 4f region[42] |
图7 (a)Ir—Ir和Ir—O配位数和反应选择性关系;(b)Ir/Ce-used催化剂Ir L3 EXAFS图;(c)Ir/Ce-used催化剂的XPS图[60]Fig.7 (a) The coordination number (CN) of Ir—Ir and Ir—O shells (data, right axis) relative to catalytic selectivity (bars, left axis) with Ir/Ce catalysts with different Ir loadings; (b) Ir L3-edge EXAFS of the Ir/Ce-used catalysts; (c) XPS analysis of the Ir/Ce-used catalysts[60] |
图8 HRTEM和EELS谱图(A~F):RR2Ti-fresh, RR2Ti-H200, RR2Ti-H300, RR2Ti-H400, RR2Ti-H500, RR2Ti-(H500+O400); (G) RR2Ti-H500样品的EELS谱图[61]Fig.8 HRTEM images and EELS spectra. (A-F) HRTEM images of RR2Ti-fresh, RR2Ti-H200, RR2Ti-H300, RR2Ti-H400, RR2Ti-H500, and RR2Ti-(H500+O400); (G) EELS spectra of the RR2Ti-H500 sample[61] |
| [1] |
|
| [2] |
|
| [3] |
( 徐如人, 于吉红, 闫文付. 化学进展, 2020, 32(8):1017.).
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
/
| 〈 |
|
〉 |