

Stimuli-Responsive Polymer Microneedle System for Transdermal Drug Delivery
Received date: 2022-07-11
Revised date: 2023-03-22
Online published: 2023-04-25
Supported by
Provincial and ministerial collaborative innovation Center project(XTCXC-202101)
Collaborative Innovation Fund of Shanghai Institute of Technology(XTCX2022-30)
Compared with oral administration and injection administration, the microneedle transdermal delivery system has the characteristics of high efficiency, safety and painless administration. In particular, the stimuli-responsive polymer microneedle systems exhibit good biocompatibility and can be realized according to the micro-changes in the environment. The function of percutaneous local and systemic intelligent drug delivery in time and space is currently an international frontier research topic. This paper focuses on the research of stimulus-responsive polymer microneedles at home and abroad in the past ten years, and focuses on the evolution of polymer microneedles, the types of internal and external environmental stimulus response and its response structure-activity mechanism. In addition, the preparation and characterization of microneedles and the application of microneedle system in the fields of biomedicine delivery, tissue and organs, dermatology and medical beauty are described in detail. The stimulation-responsive polymer microneedle system has the advantages of simple use, adjustable mechanical properties and precise targeted drug delivery, which has great research significance in the field of percutaneous targeted drug delivery. In the future, the biological in vivo load and industrial application of standardization are the direction of continuous efforts and progress of researchers.
1 Introduction
2 Preparation process and characterization methods of stimuli-responsive polymer microneedles
2.1 Preparation
2.2 Methods for characterizing the properties of polymer microneedle systems
3 Classification of stimulus-responsive polymer microneedles
3.1 Polymer microneedle system triggered by external environmental stimuli
3.2 Polymer microneedle system triggered by in vivo physiological signal stimuli
4 Stimuli-responsive polymer microneedles for transdermal delivery
4.1 Biopharmaceutical delivery
4.2 Tissue organ therapy
4.3 Detection and sensing device
4.4 Extraction of samples
4.5 Dermatology and cosmetics
5 Conclusion and outlook

Wanping Zhang , Ningning Liu , Qianjie Zhang , Wen Jiang , Zixin Wang , Dongmei Zhang . Stimuli-Responsive Polymer Microneedle System for Transdermal Drug Delivery[J]. Progress in Chemistry, 2023 , 35(5) : 735 -756 . DOI: 10.7536/PC220710
图2 微针的制备方法:(A,B) 减压浇筑法,(A) 利用浓度差制备微针的流程示意图[20]; 使用DPFM制造微针的流程示意图 (B-i); 微针的电子显微图像 (B-ii)[21]; (C) 热压花法[22],热压花制作示意图 (C-i); 微针的扫描电镜图像 (C-ii); (D) 浸渍包覆法,涂层微针制作工艺的示意图[24]; (E,F) 3D打印法[26],(E) 利用FDM3D打印和化学刻蚀技术制作微针的示意图; 制造微针 (F-i) 和在KOH溶液中腐蚀之后 (F-ii) 的光学图像; 制造的微针 (F-iii) 和在KOH溶液中腐蚀之后 (F-iv) 的扫描电子显微镜图像Fig. 2 Preparation method of microneedles. (A,B) Decompression pouring method. (A) Schematic diagram of the process for preparation of microneedles using concentration differences[20]; Schematic diagram of the process for manufacturing microneedles using DPFM (B-i); Electron microscopic images of microneedles (B-ii)[21]; (C) Hot embossing[22]. Schematic diagram of hot embossing (C-i); Scanning electron microscopic images of microneedles (C-ii); (D) Impregnation and coating method. Schematic diagram of the coating microneedle fabrication process[24]; (E,F) 3D printing method[26]. (E) The schematic illustration of the fabrication of MNs by FDM 3D printing and chemical etching; Optical images of MNs as FDM-fabricated (F-i) and after etching in KOH solution (Fii); SEM images of MNs as fabricated (F-iii) and after etching in KOH solution (F-iv) |
图3 微针系统性能表征方法。(A,B) 微针系统的力学性能[28]。(A) 应力建模示意图 (H: 微针的初始高度; H': 微针缺失尖端的高度; r1: 四边形微针底座的一半边长; r2: 四边形微针尖端的初始半侧长度; δ: 微针的位移; rδ: 压缩位移为δ时,接触面的半边长); (B) 不同类型的微针下位移变化 (*: p < 0.05;**: p < 0.01;***: p < 0.001); (C,D) 微针系统的药物递送性能。(C) 微针给药方式[31]; 不同治疗模式下的抑菌率(D-i); SA、PIL-MN和SA-PIL-MN在小鼠模型中的皮肤痤疮治疗的示意图(D-ii)[32]Fig. 3 Characterization of polymer microneedle systems. (A,B) Mechanical properties of the microneedle system[28]. (A) Schematic diagram of stress modeling (H: initial height of microneedles; H': height of microneedle missing tip; r1: half the side length of the quadrilateral microneedle base; r2: initial half length of the tip of the quadrilateral micro pin; δ: displacement of microneedle; rδ: when the compression displacement is δ, the half length of the contact surface); (B) Displacement changes under different types of microneedles (* : p < 0.05;** : p < 0.01;*** : p < 0.001); (C,D) Drug delivery performance of the microneedle system. (C) microneedle administration method[31]; Bacteriostatic rate under different treatment modes (D-i); Schematic representation of SA, PIL-MN and SA-PIL-Mn for skin acne treatment in a mouse model (D-ii)[32] |
图5 外界环境刺激响应性聚合物微针。(A) 光响应性聚合物微针系统:5-Fu-ICG-MPEG-PCL的制备示意图 (A-i); 各组A431荷瘤小鼠的生长曲线 (平均值±标准偏差 (n=5) “**” 表示P<0.01) (A-ii); 1 W/cm2 808nm激光照射5 min后A431荷瘤小鼠的近红外光热效应 (A-iii)[40]; (B) 电响应性聚合物微针系统:MXene微针生物传感器测量的方式和信号处理后的电位峰值 (B-i); MXene微针生物传感器应用 (B-ii)[45]; (C) 磁响应性聚合物微针系统:带有多层MN贴片的磁性驱动胶囊 (C-i); 使用磁力驱动胶囊将微针贴片递送至靶病变的过程 (C-ii)[50]; (D) 热响应性聚合物微针系统。用于控制透皮给药的明胶-PNIPAm微针意图 (D-i); 不同浓度的H2O2中药物释放速率图 (D-ii); 在37 ℃ RS-GP微针(1)、室温下RS-GP微针 (2)、 37 ℃ 普通明胶微针(3) 下的体外释放速率图 (D-iii)[52]; (E) 机械力响应性聚合物微针系统:装有药物的可穿戴设备与微针阵列贴集成 (E-i); 在不同治疗方式下小鼠血糖水平变化 (E-ii)[57]Fig. 5 Environmentally responsive polymer microneedles. (A) Photoresponsive polymer microneedle system. Schematic diagram of the preparation of 5-Fu-ICG-MPEG-PCL (A-i); Growth curves of A431 tumor-bearing mice in each group (mean ± standard deviation (n=5) “**” denotes P < 0.01) (A-ii); Near-infrared thermal effect of A431 tumor-bearing mice irradiated with 1 W/cm2 808nm laser for 5 min (A-iii)[40]; (B) Electrically responsive polymer microneedle system. MXene microneedle biosensor measurement method and signal processing potential peak (B-i); MXene microneedle biosensor application (B-ii)[45] ; (C) Magnetically responsive polymer microneedle system. magnetically driven capsules with multi-layer MN patches (C-i); The process of using a magnetically driven capsule to deliver a microneedle patch to a target lesion (C-ii)[50]; (D) Thermal responsive polymer microneedle system. Intent of gelatin-PNIPam microneedles for control of transdermal drug delivery (D-i); Drug release rates in different concentrations of H2O2 (D-ii); In vitro release rates of RS-GP microneedles at 37 ℃ (1), room temperature (2) and common gelatin microneedles at 37 ℃ (3) (D-iii)[52]; (E) Mechanical force responsive polymer microneedle system. Integration of medicine-equipped wearable devices with microneedle array patches (E-i); Changes in blood glucose levels of mice under different treatment methods (E-ii)[57] |
图6 体内生理信号刺激响应性聚合物微针。(A) pH响应性聚合物微针系统:ZIF-8包封微针的示意图 (A-i); 不同pH条件下CCM释放曲线 (A-ii)[67]; (B) 葡萄糖响应性聚合物微针系统:GRS胰高血糖素递送系统的机制和体外性能 (B-i); 用贴剂治疗后糖尿病小鼠的血浆胰高血糖素浓度变化 (B-ii)[70]; (C) 活性氧响应性聚合物微针系统:微针响应H2O2递释MTX治疗银屑病机理图 (C-i); 在不同浓度H2O2下微针体外释放EGCG速率图 (C-ii)[74]; (D) 酶响应性聚合物微针系统:微针溶解感染伤口清除治疗生物膜的机理示意图 (D-i); 不同pH值和温度下的左旋释放曲线 (D-ii)[82]Fig. 6 Stimulus-responsive polymer microneedles for physiological signals in vivo. (A) pH-responsive polymer microneedle system. Schematic diagram of ZIF-8 encapsulated microneedles (A-i); CCM release curves under different pH conditions (A-ii)[67]; (B) Glucose-responsive polymer microneedle system. The mechanism and in vitro performance of GRS glucagon delivery system (B-i); Changes in plasma glucagon concentrations in diabetic mice treated with the patch (B-ii)[70]; (C) Reactive oxygen reactive polymer microneedle system. Mechanism diagram of microneedles in response to H2O2 delivered MTX in the treatment of psoriasis (C-i); EGCG release rate of microneedles in vitro at different concentrations of H2O2 (C-ii)[74]; (D) Enzyme-responsive polymer microneedle system. Schematic diagram of the mechanism of microneedle lysis of biofilms in infected wound removal (D-i); left-handed release curves at different pH values and temperatures (D-ii)[82] |
表1 不同类型的刺激响应性聚合物微针系统及应用Table 1 Different types of stimulus-responsive polymer microneedle system and applications |
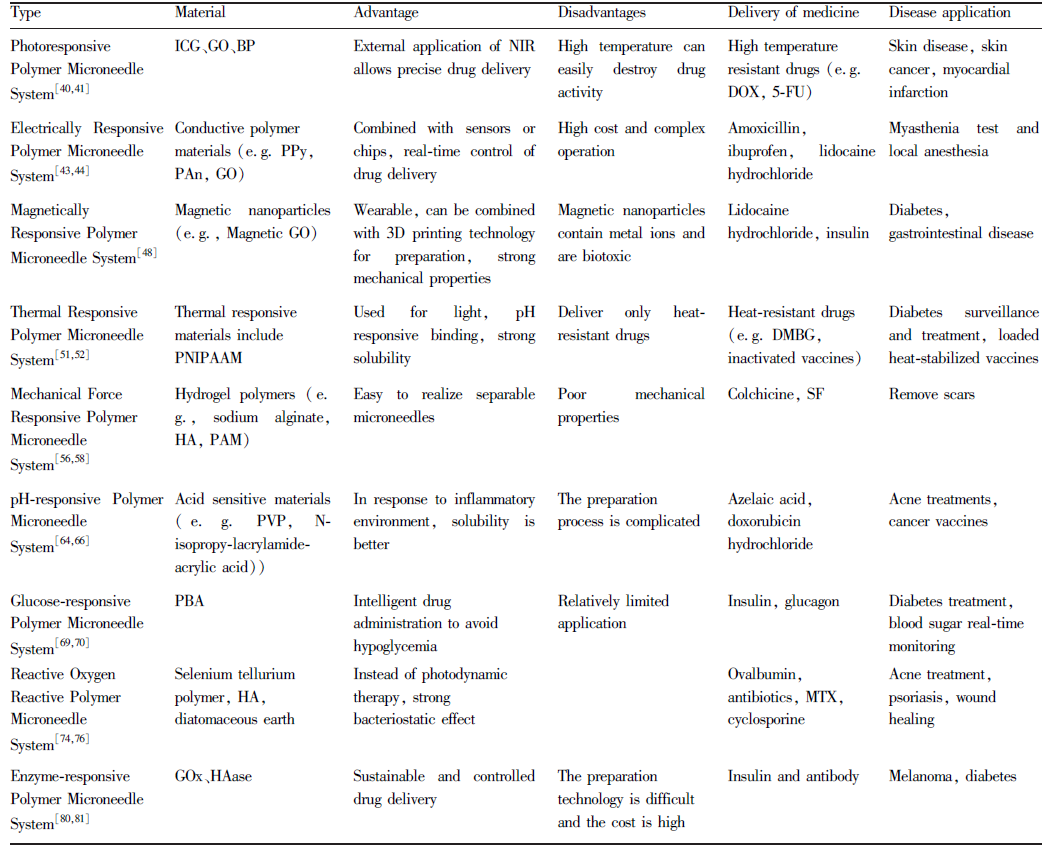 |
NoteICG: Indocyanine Green; GO: Graphene Oxide; BP: Black Phosphorus; PPy: Polypyrrole; PAn: Polyaniline; PNIPAAM: Poly (N-Isopropylacrylamide) Polymer; HA: Hualuronic Acid; PAM: Polyacrylamids; PVP: Polyvinyl Pyrrolidone; PBA: PhB(OH)2; GOx: Glucose Oxidase; HAase: Hyaluronidase; DOX: Doxorubicin Hydrochloride; 5-FU: 5-Fluorouraci, C4H3FN2O2; DMBG: Metformin; SF: Silk Fibroin; MTX: Methotrexate |
图8 刺激响应性聚合物微针经皮递释应用。(A) 组织器官治疗应用:1 W/cm2 NIR照射2 min前后, MN施加到大鼠背部皮肤的热图像 (A-i); 不同组 (对照组、BP组、BP + Hb组和BP + Hb + NIR组) 的氧释放情况 (A-ii); 第0、3、5、7和9 d不同组皮肤伤口的代表性照片(A-iii)[88]; (B) 检测及传感装置应用:葡萄糖传感器的制造工艺和检测机理图 (B-i); 在0.75 V下连续添加0.2 mM葡萄糖的PBS中的I (A)-t响应曲线 (B-ii); 0.75 V下连续添加0.5 mM葡萄糖、0.025 mM AA、0.5 mM葡萄糖、1 mM尿素和0.025 mM Gly、0.5 mM葡萄糖、0.025 mM AP (B-iii)[91]; (C) 样品提取应用:常规CTAB提取和MN提取示意图 (C-i); 微针贴片穿刺和切断后番茄叶的图像 (C-ii); 微针提取方法和CTAB提取方法作用下的DNA提取量 (C-iii)[93]; (D) 美白及抗衰应用:在对照组、HA微针、明胶微针作用下的皮肤发组织学图像 (D-i); 使用微针前后4周内皮肤弹性变化率 (P<0.05) (D-ii)[99]; (E) 防脱发应用:聚乳酸-乙醇酸接枝透明质酸 (HA-PLGA) 的合成 (E-i); 累积释放曲线(MXD溶液(●)、PLGA/MXD-NP(■)、HA-PLGA/MXD-NP(▲) ) (E-ii)[100]Fig. 8 Transdermal delivery of stimulus-responsive polymer microneedles. (A) Tissue and organ therapy. Thermal images of MN applied to the back skin of rats before and after 1 W/cm2 NIR irradiation for 2 min (A-i); Oxygen release in different groups (control group, BP group, BP + Hb group and BP + Hb + NIR group) (A-ii);Representative photographs of different groups of skin wounds on days 0, 3, 5, 7 and 9 (A-iii)[88](B) Detection and sensing device application[91]. Diagram of the manufacturing process and detection mechanism of the glucose sensor (B-i); I(A)-t response curve in stirred PBS continuously supplemented with 0.2 mM glucose at 0.75 V (B-ii); 0.5 mM glucose, 0.025 mM AA, 0.5 mM glucose, 1 mM urea, 0.025 mM Gly, 0.5 mM glucose and 0.025 mM AP were added continuously at 0.75 V (B-iii); (C) Sample extraction application. Schematic diagram of conventional CTAB extraction and MN extraction (C-i); Image of tomato leaves after puncture and cut of microneedle patch (C-ii); The amount of DNA extracted by microneedle extraction method and CTAB extraction method (C-iii)[93]; (D) Whitening and anti-aging applications. Histological images of skin hair treated with control, HA and gelatin microneedles (D-i); Change rate of skin elasticity within 4 weeks before and after microneedle application (P<0.05) (D-ii)[99]; (E) Anti-hair loss application. synthesis of polylactic acid-glycolate grafted hyaluronic acid (HA-PLGA) (E-i)[100]; the cumulative release curve (MXD solution (●), PLGA/MXD-NP (■), the HA-PLGA/MXD-NP (▲)) (E-ii) |
| [1] |
(陈明龙, 杨丹, 孙颖, 权桂兰, 吴传斌, 潘昕. 药学进展, 2020, 44(5): 324. ).
|
| [2] |
|
| [3] |
|
| [4] |
(陈永杭, 李欣芳, 余伟江, 王幽香. 化学进展, 2021, 33(07): 1152).
|
| [5] |
|
| [6] |
|
| [7] |
Ullah, Khan, Choi, Kim. Polymers, 2019, 11(11): 1834.
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
(陈怡, 宋婷, 陈雯琳, 李海梁, 卢爱玉, 张峻颖. 药学研究, 2021, 40(11): 744.).
|
| [12] |
(张朵朵, 吴艳丽, 鞠大宏, 刘梅洁, 郝保华. 中华中医药杂志, 2014, 29(8): 2559.).
|
| [13] |
|
| [14] |
(赵笑, 李欣芳, 张鹏, 王幽香. 化学进展, 2017, 29(12): 1518.).
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
(杨倩丽, 康晓明, 孙静, 魏柳荷, 马志. 化工进展, 2015, 34(08): 3075).
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
(蔡紫煊, 张斌, 姜丽阳, 许国贺, 马晶军. 化学进展, 2019, 31(12): 1653.).
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
/
| 〈 |
|
〉 |