

Immunity and Aptamer Biosensors for Cocaine Detection
Received date: 2022-09-19
Revised date: 2022-11-13
Online published: 2023-04-30
Supported by
National Natural Science Foundation of China(21974068)
National Natural Science Foundation of China(21890740)
National Natural Science Foundation of China(21890742)
National Natural Science Foundation of China(9195401)
Cocaine has become one of the most dangerous and illicitly abused drugs today due to the adverse effects of long-term cocaine abuse, such as arrhythmia, myocardial infarction, stroke, hypertension and aortic stiffness. Traditional cocaine chromatographic analysis methods have disadvantages such as time-consuming, cumbersome sample processing and complicated operations. Therefore, improving cocaine detection methods has a certain positive impact on crime-fighting and medicine-developing. Due to the accuracy and portability of biosensors, immunological and aptamer technologies for specific capture of targets have become an important direction for cocaine detection. In this review, different types of cocaine biosensors in recent years are mainly described, covering the research progress of cocaine detection based on electrochemical, fluorescence, colorimetric and other methods. The immuno- and aptamer-based biosensors of cocaine are reviewed, the advantages, disadvantages and development directions of cocaine sensors are summarized.
1 Introduction
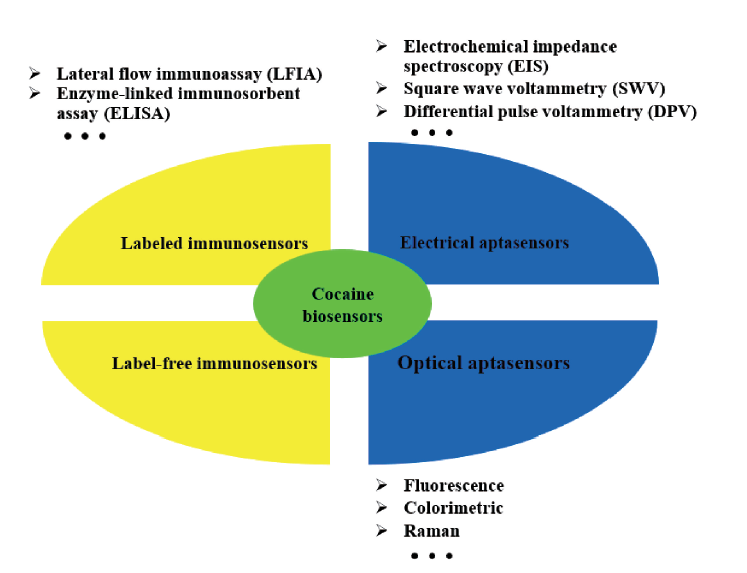
2 Immunosensors for cocaine detection
2.1 Labeled immunosensors
2.2 Label-free immunosensors
3 Aptasensors for cocaine detection
3.1 Fluorescent aptasensors
3.2 Colorimetric aptasensors
3.3 Electrochemical aptasensors
3.4 Other aptasensors
4 Conclusion and outlook
Gehui Chen , Nan Ma , Shuaibing Yu , Jiao Wang , Jinming Kong , Xueji Zhang . Immunity and Aptamer Biosensors for Cocaine Detection[J]. Progress in Chemistry, 2023 , 35(5) : 757 -770 . DOI: 10.7536/PC220916
表1 不同可卡因免疫传感器及其检出限对比Table 1 A comparison of different cocaine immunosensors and their limits of detection |
| Approach of detection | Used sample | Linear detection range (mol/L) | Limit of detection (mol/L) | ref |
|---|---|---|---|---|
| Electrochemical-based ELISA | Water/Saliva/Urine | — | 4.95×10-13 | 19 |
| Colorimetric Immuno-microarray | Oral fluids | 3.63×10-9~9.9×10-7 | 3.63×10-9 | 21 |
| LFIA | Urine | 1.65×10-8~1.65×10-6 | 1.65×10-8 | 26 |
| LFIA | Saliva | 1.65×10-8~3.30×10-6 | 1.62×10-9 | 27 |
| Electrochemical | Urine/Sweat/Saliva/Serum | 1.65×10-8~8.25×10-7 | 1.19×10-8 | 30 |
| Fluorescence | PBS buffer | — | 2.30×10-11 | 31 |
| Electrochemical | PBS buffer | 0.50×10-6~2.50×10-5 | — | 34 |
| SHG | PBS buffer | — | 7.5×10-11 | 35 |
表2 不同可卡因适配体传感器及其检出限对比Table 2 A comparison of different cocaine aptasensors and their detection limits |
| Method | Linear range (mol/L) | Detection limit (mol/L) | ref |
|---|---|---|---|
| Fluorescence | — | 5×10-6 | 62 |
| Fluorescence anisotropy | — | — | 63 |
| Fluorescence | 0~1×10-5 | 5×10-8 (in 10% saliva) | 64 |
| Fluorescence | 5×10-10~8×10-8 | 8.4×10-11 | 65 |
| Fluorescence | 0~1×10-10 | 5.4×10-13 | 66 |
| Cas-12a based fluorescence | 4.7×10-7~1.5×10-2 | 3.4×10-7 | 67 |
| EWF-based fluorescence | 1×10-5~5×10-3 | 1.05×10-5 | 68 |
| Fluorescence | 1×10-6~5×10-4 | 2.5×10-7 | 69 |
| Fluorescence | 1×10-7~1×10-4 | 4.6×10-9 | 72 |
| Fluorescence | 1×10-8~1×10-4 | 8×10-10 | 73 |
| Colorimetric | — | 8.25×10-9 mol (visual) 7.79×10-9 mol (camera) | 75 |
| Colorimetric | 2×10-10~2.5×10-8 | 9.7×10-10 | 76 |
| Colorimetric | — | 1.32×10-8 mol (visual) 1.17×10-8 mol (camera) | 77 |
| Colorimetric | 0~1×10-6 | 7.49×10-9 | 78 |
| Colorimetric | 1×10-9~1.5×10-7 | 5×10-10 | 79 |
| Colorimetric | 1×10-8~1.5×10-7 | 3.3×10-9 | 80 |
| Colorimetric | 2×10-9~1×10-7 | 4.4×10-10 | 81 |
| Colorimetric | — | 1×10-5 | 82 |
| Colorimetric | 1×10-5~5×10-3 | 5×10-5 (in urine) 2×10-4 (in sweat) | 83 |
| SWV | 5×10-8~1×10-6 and 1×10-6~3.5×10-5 | 2.1×10-8 | 86 |
| SWV | — | — | 87 |
| EIS/DPV | 3.3×10-12~3.3×10-9 | 1.29×10-12 (EIS) 2.22×10-12 (DPV) | 89 |
| EIS | 1×10-15~1×10-12 and 1×10-12~1×10-7 | 3.33×10-16 | 90 |
| EIS | 9×10-11~8.5×10-8 | 2.9×10-11 | 91 |
| DPV | 3.3×10-10~3.3×10-5 | 1×10-10 | 92 |
| SWV | 3.3×10-11~3.3×10-6 | 9×10-12 | 93 |
| DPV | 1×10-11~7×10-11 | 2.6×10-13 | 94 |
| DPV | 4×10-11~1.5×10-7 | 1.5×10-11 | 95 |
| EMPAS | 2×10-6~5×10-5 | 9×10-7 | 96 |
| EMPAS | 5×10-7~5×10-6 | 3×10-7 | 97 |
| Interfacial capacitance sensing | 1.45×10-14~1.45×10-11 | 7.8×10-15 | 98 |
| FET | — | 1×10-9 | 99 |
| Conductance change | 1×10-9~1×10-5 | 1×10-9 | 102 |
| α-HL nanopore | 5×10-8~1×10-4 | 5×10-8 | 103 |
| Personal glucometer | 1×10-8~6×10-7 | 5.2×10-9 | 104 |
| LC optical sensor | 1×10-9~1×10-5 | 1×10-9 | 106 |
| LC optical sensor | 1×10-10~1×10-5 | — | 108 |
| LPFG | 2.5×10-5~7.5×10-5 | 2.5×10-5 | 109 |
| PIERS | 5×10-9~1×10-5 | 5×10-9 | 110 |
| ECL | 1×10-10~1×10-7 | 6×10-11 | 111 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
/
| 〈 |
|
〉 |