

Study on the High-Voltage Resistance of Layered Oxide Cathode Materials for Sodium-Ion Batteries
Received date: 2023-10-30
Revised date: 2024-02-17
Online published: 2024-03-15
Supported by
National Natural Science Foundation of China(52072274)
National Natural Science Foundation of China(52272021)
National Natural Science Foundation of China(52232002)
Knowledge Innovation Project of Wuhan, China(2023020201010131)
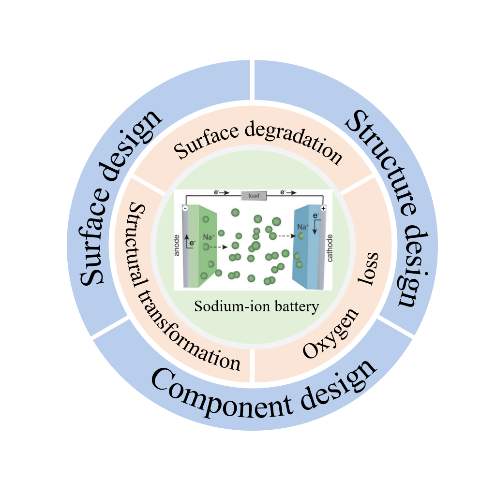
sodium-ion batteries have shown great application prospects in the field of large-scale energy storage and low-speed electric vehicles due to their resource and cost advantages.Among various cathodes reported,layered oxide cathode materials have been widely investigated owing to their high theoretical capacity and simple synthesis method.However,many adverse reactions and phenomena such as structural instability and surface degeneration are prone to occur during its cycling process,especially at high voltage,which hinders its application in commerce.This article briefly reviews the mechanism of structural transformation,surface degradation,and oxygen loss of layered oxide cathode at high-voltage,focuses on the strategies to improve the high-voltage resistance of layered oxide cathode,and aims to provide reasonable insights for improving the high-voltage stabilization of layered oxide cathode materials and designing sodium-ion layered oxide cathode materials with high performance.Finally,the shortcomings of sodium-ion battery layered oxide cathode materials in modification and future research directions are also summarized。
1 Introduction
2 The degradation mechanism of layered oxide cathode materials at high voltage
3 Component design to improve the high-voltage resistance of layered oxide cathode
3.1 Cationic doping
3.2 Anionic doping
3.3 Cation and anion doping
4 Surface design to improve the high-voltage resistance of layered oxide cathode
5 Structural design to improve the high-voltage resistance of layered oxide cathode
5.1 Composite phase
5.2 Microstructure design
6 Conclusion and outlook
Lixiang Ding , Xuke Li , Xuefeng Liu , Yimin Liu , Wen Lei , Haijun Zhang . Study on the High-Voltage Resistance of Layered Oxide Cathode Materials for Sodium-Ion Batteries[J]. Progress in Chemistry, 2024 , 36(7) : 987 -997 . DOI: 10.7536/PC231023
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
/
| 〈 |
|
〉 |