

Research Progress of Ecotoxicology of PPCP Pollutants
Received date: 2024-01-30
Revised date: 2024-04-04
Online published: 2024-04-16
Supported by
National Natural Science Foundation of China(42322707)
National Natural Science Foundation of China(42077223)
National Natural Science Foundation of China(42277266)
GuangDong Basic and Applied Basic Research Foundation(2023A1515140118)
Pharmaceuticals and personal care products(PPCPs)are a large category of emerging pollutants that have been highly concern in recent years.the huge production and rapid consumption demand of PPCPs make them widely enter and highly exist in various environmental mediums.Due to migration,transformation and bioaccumulation,PPCPs enter the ecological environment,causing different degrees of negative impact on organisms and human bodies,thus bringing serious threats to the ecological environment and human health.in this review,we summarize the exposure sources,pathways and characteristics of current PPCPs in the environment,conclude the degradation method and pathway of PPCPs in the environment,review the main biotoxicity of PPCPs,overview the exposure concentrations and the health influences on the human body,and finally have some outlooks on the research field of ecotoxicity of PPCPs。
1 Introduction
2 Environmental exposure of PPCPs
2.1 Environment sources and migration and transform pathway of PPCPs
2.2 Environment concentration and distribution of PPCPs
3 Degradation method and mechanism of PPCPs
4 Biotoxicity of PPCPs
4.1 Acute toxicity
4.2 Neurotoxicity
4.3 Reproductive and developmental toxicity
4.4 Endocrine disruption
4.5 Immunotoxicity
4.6 Cardiovascular toxicity
4.7 Other toxicities
5 Human health risk of PPCPs
5.1 Human exposure to PPCPs
5.2 Human health risk/assessment
6 Conclusion and outlook
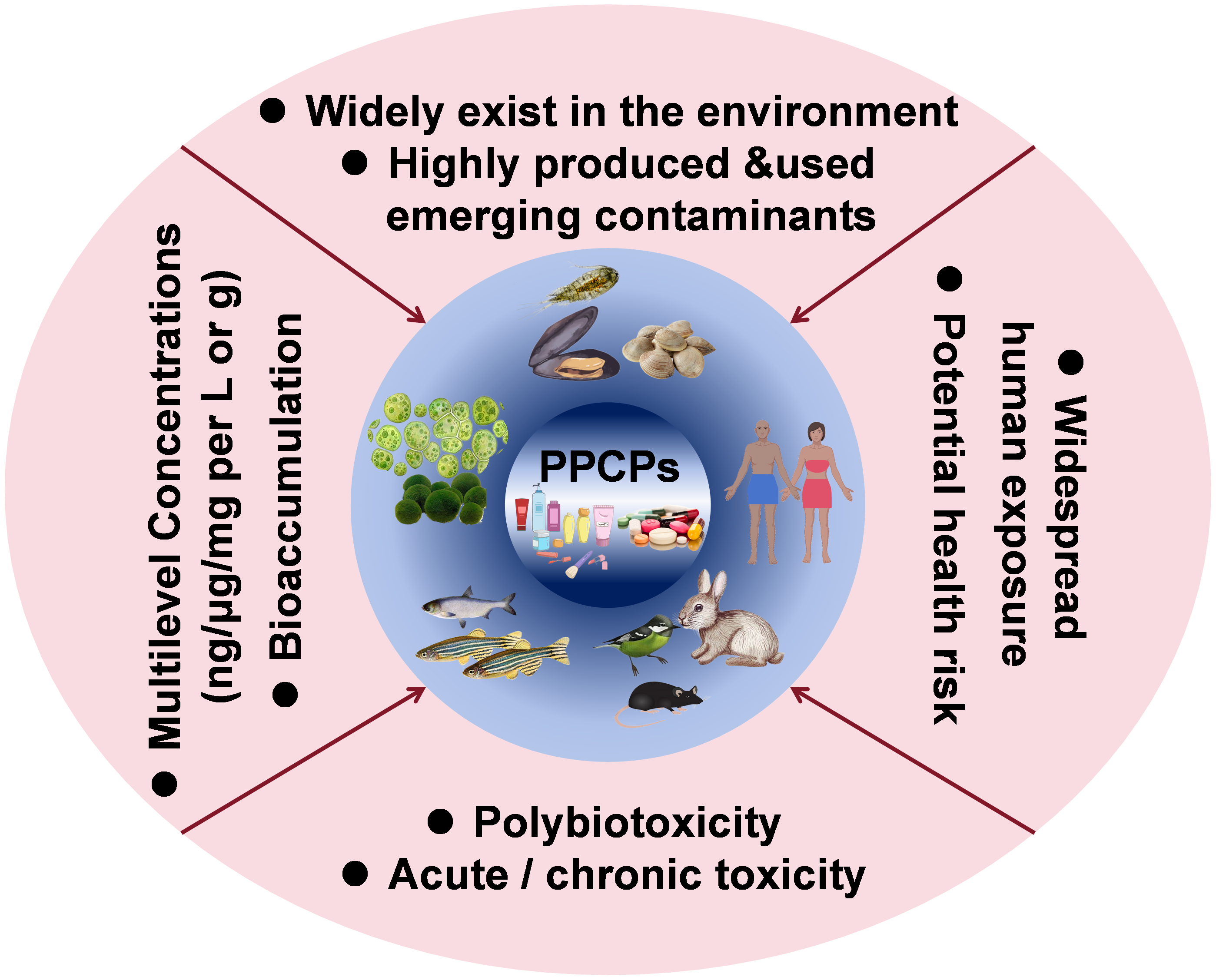
Chuanzi Gao , Haolin Liao , Yibo Wang , Yi Zheng , Chunmiao Zheng , Wenhui Qiu . Research Progress of Ecotoxicology of PPCP Pollutants[J]. Progress in Chemistry, 2024 , 36(9) : 1363 -1379 . DOI: 10.7536/PC240127
表1 Environmental exposure concentration of PPCPsTable 1 Concentrations of PPCPs in the environment |
| Sample classification | Medium | Location | Concentration of PPCPs a | Ref |
|---|---|---|---|---|
| Aquatic environment | Surface water | China | Sulfamethoxazole (ND b~57.76 ng/L);Sulfamethoxazole (28.34 ng/L, median);Ofloxacin (114 ng/L, median);Norfloxacin (152 ng/L, median);Erythromycin (34 ng/L, median); Erythromycin (0~722.04 ng/L);Roxithromycin (53 ng/L, median); Roxithromycin (3.63 ng/L, max); Tetracycline (113.89 ng/L, max);Tetracycline (0~9.74 ng/L) | 39,40 56,57 |
| UK | 4-tert-octylphenol (ND~1 293 000 ng/L); Chloroxylenol (ND~358000 ng/L) | 8 | ||
| Canada | Galaxolide fragrance(HHCB) (0.031 ng/m3, mean) | 58 | ||
| Sri Lanka | Sulfamethoxazole (ND~934 ng/L) | 41 | ||
| Italy | Sulfamethoxazole (0.7~16 ng/L) | 42 | ||
| USA | Sulfamethoxazole (77.7 ng/L, max) | 43 | ||
| Underground water | China | Sulfamethoxazole (ND~91 ng/L); Sulfamethazine (ND~269.7 ng/L); SMX (ND~14.2 ng/L) Sulfadiazine (29.9 ng/L, mean); Ciprofloxzcin (4~9.68 ng/L); Ciprofloxzcin (0.82 ng/L, mean) Tetracycline (2.26~9.51 ng/L); Erythromycin (1.47~13.8 ng/L); Norfloxacin (4.74~52.6 ng/L) Oxytetracycline (1.1~7.24 ng/L) | 44 59 60 | |
| USA | Lincomycin (0.32 ng/L, max); Sulfamethoxazole (1.11 ng/L, max) | 45 | ||
| Spain | Sulfamethoxazole (23.4 ng/L, mean); Sulfadiazine (11.62 ng/L, mean) | 46 | ||
| Sediment | China | Sulfamethoxazole (1.27~688.59 ng/L); Sulfamethoxazole (0~11.3 ng/L); Sulfadiazine (0~0.41 ng/L); Oxytetracycline (0~8.73 ng/L); Ciprofloxzcin (0~15.33 ng/L); Ofloxacin (0.9~18.27 ng/L); Erythromycin (0.15-3.96 ng/L) | 39,47 | |
| Pore water | China | Erythromycin (29.9 ng/L, mean); Lincomycin (20.2 ng/L, mean); Oxytetracycline (47.8 ng/L, mean); Ofloxacin (33.6 ng/L, mean) | 61,62 | |
| Rainwater | USA | Ciprofloxzcin (10.3 ng/L, max); enrofloxacin (2.97 ng/L, max) | 63 | |
| Seawater | China | Sulfachloropyridazine (0.2 ng/L, mean); Sulfadimethoxine (1.8 ng/L, mean); Sulfamethoxazole (7.7 ng/L, mean); Trimethoprim (7.2 ng/L, mean); Azithromycin (4.8 ng/L, mean); Clarithromycin (3 ng/L, mean); Erythromycin-H2O (0.5 ng/L, mean); Roxithromycin (2.7 ng/L, mean); Enoxacin (3.3 ng/L, mean); Enrofloxacin (7.2 ng/L, mean); Norfloxacin (0.7 ng/L, mean); Ofloxacin (25.4 ng/L, mean); Chloramphenicol (2.6 ng/L, mean); Florfenicol (7.7 ng/L, mean); Thiamphenicol (3.7 ng/L, mean); Penicillin G (0.4 ng/L, mean); Bisphenol analogues (206 ng/g, median); Parabens (13 ng/g, median); paraben metabolites (686 ng/g, median) | 25,64 | |
| Northern Europe | Sulfamethoxazole (42 ng/L, median); Clarithromycin (14 ng/L, median) | 48 | ||
| Greece | Sulfamethoxazole (11 ng/L, median); Clarithromycin (16 ng/L, median) | 48 | ||
| Italy | Sulfamethoxazole (7.2 ng/L, median); Clarithromycin (8.5 ng/L, median) | 48 | ||
| Wastewater | Canada | Galaxolide fragrance(HHCB) (1000~1800 ng/L); Toxalide fragrance(AHTN) (140~270 ng/L) | 58 | |
| Greece | Ibuprofen (7.0~8.9 μg/L) | 65 | ||
| Japan | Ibuprofen (816±815 μg/L, mean) | 66 | ||
| USA | Acetaminophen (0.006 μg/L, median); Benzophenone-3 (0.2 μg/L, median); Bisphenol A (0.12 μg/L, median); Caffeine (0.053 μg/L, median); Carbamazepine (0.08 μg/L, median); Codeine (0.139 μg/L, median); Diltiazem (0.049 μg/L, median); Galaxolide fragrance(HHCB) (0.28 μg/L, median); N,N-diethyl-m-toluamide (DEET) (0.18 μg/L, median); Sulfamethoxazole (0.15 μg/L, median); Toxalide fragrance (AHTN) (1 μg/L, median); Triclosan (0.25 μg/L, median); Trimethoprim (0.038 μg/L, median); 1,4-dichlorobenzene (0.110 μg/L, median) | 49 | ||
| Aquatic organism | China | parabens and metabolites (8190 ng/g, max); parabens and metabolites (1135 ng/g, max) | 25 | |
| Air | Indoor air | Canada | Galaxolide fragrance (HHCB) (0.30~18 ng/m3); Toxalide fragrance (AHTN) (0.09-17 ng/m3) | 58 |
| Outdoor air | Canada | Galaxolide fragrance (HHCB) (0.99 ng/m3, median); Toxalide fragrance (AHTN) (0.22 ng/m3, median) | 58 | |
| Indoor dust | China | Galaxolide fragrance (HHCB) (1.87~577 ng/g, median:31.4 ng/g); Musk ketone (10.4 ng/g, median); Toxalide fragrance (AHTN) (11.7 ng/g, median); Parabens (418 ng/g, GMc); siloxanes (21.5~21 000 ng/g, mean:1540±2850 ng/g) | 9,50,67 | |
| Spain | Musk ketone (14.4~2300 ng/g, median:111 ng/g); Musk xylene (133 ng/g, median) | 68 | ||
| South Korea | Parabens (2320 ng/g, GM) | 9 | ||
| Japan | Parabens (2300 ng/g, GM) | 9 | ||
| USA | Parabens (1390 ng/g, GM) | 9 | ||
| Germany | Galaxolide fragrance (HHCB); (700 ng/g, median); Toxalide fragrance (AHTN); (900 ng/g, median) | 69 | ||
| Soil | Soil | China | Carbamazepine (1.8 μg/kg,mean); Gemfibrozil (0.9 μg/kg, mean);Tetracycline (19.9 μg/kg, mean); Triclosan (1.8 μg/kg), mean ; Bisphenol A (3.4 μg/kg, mean) ; Diethyl Phthalate (0.178 μg/kg, mean) | 10,51 |
| USA | Ibuprofen (318.5 μg/kg, mean); Estriol (7.73 μg/kg, mean); Clofibric Acid (4.27 μg/kg, mean); Naproxen (23.79 μg/kg, mean); Triclosan (8.16 μg/kg, mean); Bisphenol A (31 μg/kg, mean) | 52⇓~54 |
a,the concentration without special annotation means the range;b,ND=not detected;c,GM=geometric mean。 |
表2 Chronic biological toxicity of PPCPTable 2 Chronic biotoxicity of PPCPs |
| Toxicity classification | Organism | Species | PPCPs chemicals | Effective concentration | Toxicity effect | Ref |
|---|---|---|---|---|---|---|
| Neurotoxicity | Mammal | Mice | Triclosan | 1000 mg/kg, 2000 mg/kg, 4000 mg/kg | Causes behavioral disorders in mice | 140 |
| Mice | Acetaminophen | 30 + 30 mg/kg, 4 h apart | Decline in memory, learning ability and cognitive flexibility | 94 | ||
| Fish | Gambusia affinis | Fluoxetine | 0.05~5 μg/L | Sleeping time expanded | 141 | |
| Gobiocypris rarus | Alprazolam | 10 ng/L | Disturb GPC、CHOP、Met et al. neurosubstances | 100 | ||
| Gobiocypris rarus | Lorazepam | 100 ng/L | Disturb the Cho、5-HT、Trp、5-HIAA et al. neurosubstances | |||
| Invertebrate | Clams | Caffeine | 50 μg/L | AChE activity declines 56% | 101 | |
| Carbamazepine | 0. 1 μg/L | AChE activity declines 53% | ||||
| Reproductive toxicity | Mammal | Rat | 17β-Estradiol | 10~50 mg/kg (E2) | Centrilobular hepatocellular hypertrophy: diffuse Pituitary hyperplasia; breast hyperplasia: increased number of cystic follicles in the ovaries, endometrial and endometrial glandular hypertrophy | 110 |
| Rat | 17β-Estradiol | ng/kg~mg/kg (E3, E2) | Uterine response: increased uterine weight due to water retention and cell proliferation | 111 | ||
| Fish | Juvenile | 17β-Estradiol | 0.05~0.5 mg/kg (E2) | Affects spawning | 112 | |
| Oryzias latipes | Diclofenac | 10 mg/L | Reduced egg hatchability | 142 | ||
| Oryzias latipes | Ibuprofen | 0.0001 mg/L | Reduce hatchability and yolk proteins content | 143 | ||
| Dicentrarchus | 17β-Estradiol | 10 mg/kg (E2) | Affect reproduction | 113 | ||
| Pimephales Promelas | Fadrozole | 2 μg/L 21-day exposure | Decline in reproduction rate | 144 | ||
| Pimephales promelas | Fluoxetine | 1 μg/L | Impacted mating behavior, specifically nest building and defending in male fish | 145 | ||
| Invertebrate | Daphnia magna | Propranolol | 0.128 mg/L | Reduced fecundity and reproductive rate | 146 | |
| Lumbriculus variegatus | 3-benzylidene-camphor | 44.2 μmol/L | Substantial reduction in fertility and significant increase in mortality | 114 | ||
| Chironomus riparius | Carbamazepine | 0.625 mg/kg 28-day exposure | Inhibit pupa to be formed | 109 | ||
| Developmental toxicity | Plant | Lactuca sativa | Erythromycin | 0.1~300 mg/kg | Inhibition of bud development | 147 |
| Brassica rapa chinensis | Chlortetracycline | 2.5~20 mg/kg | Inhibition of bud development | 148 | ||
| Oenanthe javanica | Oxytetracycline | 0.5~10 mg/kg | Inhibition of plant height | 149 | ||
| Solanum lycopersicum | Sulfadiazine | 0.1~300 mg/kg | Inhibition of bud development | 147 | ||
| Fish | Danio rerio | Musk xylene | 33 μg/L | Significant impact in early life stages | 150 | |
| Danio rerio | Amitriptyline | 0.001~1000 μg/L | Inhibition of growth and development; alteration of ACTH concentration level; oxidative stress | 151 | ||
| Danio rerio | Muscone | 33 μg/L | Reduced fish mass and length of females and reduced fecundity | 107 | ||
| Pimephales promelas | β-blocker Propranolol | 3.4 mg/L | Reduced body weight and egg hatchability | 152 | ||
| Algae | Scenedesmus obliquus | NSAIDs (Ibuprofen, aspirin, ketoprofen) | 107.91, 103.05, and 4.03 mg/L, respectively | Inhibition of algal growth | 153 | |
| Chlorella pyrenoidosa | Diclofenac | >100 mg/L | Alteration of chlorophyll a, lipid accumulation, and antioxidant enzyme function, thereby affecting growth | 154 | ||
| amphibian species | Bufo americanus. | Acetaminophen | 100 μg/L 28-day exposure | Survival rates influenced | 155 | |
| Rana pipiens | Acetaminophen | 1 μg/L 14-day of exposure | Behavior affected | |||
| Endocrine disruption | Invertebrate | Mytilus spp. | Gemfibrozil | 1000 μg/L | Endocrine disruption | 121 |
| Reptilia | Trachemys scripta | Estradiol | 1 μg/g | Induction of VTG (vitellogenin) | 156 | |
| Fish | Oryzias latipes | Fluoxetine | (100~500 μg/L) 28-day exposure | Affects estradiol concentrations in fish | 157 | |
| Mammal | Rat | Benzophenone 2(BP-2) | 1000 mg/kg | Causes anti-thyroid effects in the body goiter | 120 | |
| Immunotoxicity | Fish | Danio rerio | Enrofloxacin | 10 μg/L, 100 μg/L | Significant reductions in macrophage and neutrophil populations and biomarkers of immunosuppressive effects in zebrafish | 122 |
| Danio rerio | Tetracycline | 100 μg/L | Neutrophil counts were significantly reduced in offspring zebrafish | 123 | ||
| \ | \ | Butylated hydroxytoluene | \ | Often causes skin, eye, and lung irritation and toxic to immune system | 124 | |
| Invertebrate | Clams | Fluoxetine | 1 μg/L, 5 μg/L | Influenced immunological parameters and Acetylcholinesterase (AChE) decreased significantly | 125 | |
| Cardiovascular toxicity | Mammal | Human | Caffeine | \ | Correlation with cardiovascular disease (CVD) | 126 |
| Beagle dogs | Sibutramine | 30 mg/kg | Increasing Heart Rate and Blood Pressure | 128 | ||
| Invertebrate | Manila Clam (Venerupis philippinarum) | Caffeine, ibuprofen, carbamazepine, novobiocin | Caffeine =15 μg/L, Ibuprofen =10 μg/L, Carbamazepine =1 μg/L, Novobiocin =1 μg/L | Decrease the blood cell viability | 158 | |
| Manila Clam | caffeine | 15 μg/L | Decreased blood cell viability | 4 | ||
| Fish | S.trutta f.fario | Water-borne diclofenac | 5~50 μg/L | Affects integrity of kidney and gill | 129 | |
| Gasterosteus aculeatus | Naproxen | 299 and 1232 μg/L | Increased renal hematopoietic hyperplasia | 130 | ||
| Other toxicity | Birds | Domestic fowl | Diclofenac | 9.8 mg/kg | Showed signs of gout with deposits of urates in the kidneys, liver, heart and spleen | 131 |
| Mammal | Rat | Triclosan | 30~250mg/kg | Liposynthesis was affected, and TG levels of serum, liver, and adipose tissue were reduced | 132 | |
| Rat | Propranolol and atenolol | 5, 10, and 20 μg/mL | Induction of reactive oxygen species and mitochondrial damage to cardiac tissue | 133 | ||
| Rat | Mesalazine | 25, 50, 100 μM | Triggers overproduction of mitochondrial ROS, releases cytochrome c, and causes cardiotoxic effects | 134 | ||
| Invertebrate | Daphnia magna | Indomethacin, ibuprofen | 1 mg/L | Reduced feeding rate and modulated activities of key enzymes like Alkalineand acid phosphatases, lipase, peptidase, β-galactosidase, and glutathione stransferase | 135 | |
| Mytilus galloprovincialis | Propranolol, Acetaminophen | Propranolol (11 microg/L), Acetaminophen (23 and 403 μg/L) | Modulates antioxidant enzyme activity and induces oxidative stress | 136 | ||
| Mytilus galloprovincialis | Carbamazepine, ibuprofen, fluoxetine | 0.05~500 ng/g | Regulates enzyme activities indicative of oxidative stress and DNA damage | 102 | ||
| Mytilus galloprovincialis | Ibuprofen | 250 ng/L | Affects the arachidonic pathway | 137 | ||
| Algae | Chlorella vulgaris, Microcystis aeruginosa | Ser-HCL | 25~200 μg/L | Altered the composition of photosynthetic community. | 138 | |
| Chlorella vulgaris, Desmodesmus armatus. | Bisoprolol and Ketoprofen | 100 mg/L | Regulation of antioxidant enzyme activity, cell morphology, chlorophyll content | 139 |
表3 Human exposure concentration of PPCPsTable 3 Concentrations of PPCPs in human |
| Medium | Location | Concentration of PPCPs a | Ref |
|---|---|---|---|
| Plasma | China | Galaxolide fragrance (HHCB) ( <8~125.9 ng/g lwb); Toxalide fragrance (AHTN) ( <8~2.7 ng/g lw); Musk xylene ( <8~69.0 ng/g lw); Musk ketone ( <8~60.6 ng/g lw); Galaxolide fragrance (HHCB ) ( <8~314.7 ng/g lw); Toxalide fragrance (AHTN) ( <8~39.5 ng/g lw); Musk xylene ( <8~39.0 ng/g lw); Musk ketone (<8~43.5 ng/g lw); | 161 |
| South Korea | Galaxolide fragrance (HHCB) (170~1400 ng/g lw); Toxalide fragrance (AHTN) (<170~1400 ng/g lw); Musk xylene (170~510 ng/g lw); Musk ketone (<170 ng/g lw); Galaxolide fragrance (HHCB) (<670~2700 ng/g lw); Toxalide fragrance(AHTN) (670~2700 ng/g lw); Musk xylene (<670 ng/g lw); Musk ketone (<670 ng/g lw); | 178 | |
| USA | Galaxolide fragrance (HHCB) (380~1700 ng/L) | 179 | |
| Austria | Galaxolide fragrance (HHCB) (450 ng/L, median); Musk xylene (11 ng/L, median) | 159 | |
| Italy | Daphomycin (19~199 μg/mL) | 180 | |
| Breast milk | China | Galaxolide fragrance(HHCB) (4.42~58.2 ng/g lw) | 181 |
| China | Toxalide fragrance (AHTN) (3.33~29 ng/g lw); Musk ketone (<1.40~10.71 ng/g lw); Triclosan (0.41~0.77 μg/kg); Triclocarban (0.03~4.28 μg/kg); Galaxolide fragrance (HHCB) (92 ± 70 ng/g, mean)Tonalide (16 ± 12 ng/g, mean)Musk xylene (26 ± 22 ng/g, mean)Musk ketone (16 ± 14 ng/g, mean) | 181⇓~183 | |
| Italy | Daphomycin (0.12~0.32 μg/mL) | 180 | |
| Sweden | Galaxolide fragrance (HHCB) (63. 9 ng/g, median); Tonalide (10.4 ng/g, median) | 165 | |
| Denmark | Galaxolide fragrance (HHCB) (38~422 ng/g); Toxalide fragrance (AHTN) (5.58~37.9 ng/g lw); AHMI (nd~9.94 ng/g lw); Musk xylene (nd~46.4 ng/g lw); Musk ketone (nd~26.9 ng/g lw) | 164 | |
| Japan | Galaxolide fragrance (HHCB) (<50~440 ng/g lw); Toxalide fragrance (AHTN) (<50~190 ng/g lw) | 184 | |
| South Korea | Galaxolide fragrance (HHCB) (<5.00~1346 ng/g lw); Toxalide fragrance (AHTN) (<5.00-350 ng/g lw); Musk xylene (<2.00~73.5 ng/g lw); Musk ketone (<2.00~250 ng/g lw) | 185 | |
| Czech Republic | Galaxolide fragrance (HHCB) (13~720 ng/g lw); Toxalide fragrance (AHTN) (13~720 ng/g lw); Musk xylene (<10~156 ng/g lw); Musk ketone (<10~93 ng/g lw) | 186 | |
| USA | Galaxolide fragrance(HHCB) (<5~917 ng/g lw); Toxalide fragrance(AHTN) (<5~144 ng/g lw); Musk xylene (<2~150 ng/g lw); Musk ketone (<2~238 ng/g lw); Galaxolide fragrance(HHCB) (20.1~131.6 ng/g lw); Toxalide fragrance (AHTN) (26.4~41.4 ng/g lw) | 163 | |
| Germany | Musk xylene (10~1220 ng/g lw); Musk ketone (<10~240 ng/g lw); Galaxolide fragrance (HHCB) (21~1316 ng/g lw) Toxalide fragrance (AHTN) (16~148 ng/g lw); Musk xylene (1.3~47.9 ng/g lw); Musk ketone (2.1~82.9 ng/g lw); Galaxolide fragrance (HHCB) (16~108 ng/g lw); Toxalide fragrance (AHTN) (11~58 ng/g lw); Musk xylene (10~30 ng/g lw); Musk ketone (5~15 ng/g lw) | 187⇓~189 | |
| Fatty | Germany | Musk xylene (20~220 ng/g lw); Musk ketone (10~220 ng/g lw) | 190 |
| Switzerland | Galaxolide fragrance (HHCB) (12~171 ng/g lw); Toxalide fragrance (AHTN) (1~23 ng/g lw); Musk xylene (6,7~288 ng/g lw); Musk ketone (<1~173 ng/g lw); | 191 | |
| Italy | Galaxolide fragrance (HHCB) (361 ± 467 ng/g lw, mean); Toxalide fragrance (AHTN) (132 ± 264 ng/g lw, mean) | 192 | |
| Urine | China | Triclosan (0. 36 μg/L, mean); Triclocarban (0.36 μg/L, mean); Doxycycline (4.6 ng/mL, 95th); Azithromycin (1.5 ng/ml, 95th); Trimethoprim (0.48 ng/ml, 95th); Florfenicol (0.32 ng/mL, 95th); Amoxicillin (15.70 ng/mL, 95th); Azithromycin (7.02 ng/mL, 90th); Erythromycin (78.05 ng/ml, 95th); Clarithromycin (183 ng/mL, 95th); Ofloxacin (35.1 ng/mL, 95th); Tetracycline (10.7 ng/mL, 90th); Sulfamethoxazole (0.11 ng/mL, 95th); rimethoprim (0.23 ng/mL, 90th); Azithromycin (0.03 ng/mL, 90th); Penicillin G (2.53 ng/mL, 90th); Penicillin V (1.20 ng/mL, 95th); Tetracycline (0.18 ng/mL, 90th); Doxycycline (0.19 ng/ml, 75th); Norfloxacin (0.14 ng/mL, 95th); Florfenicol (0.10 ng/ml, 95th); Bisphenol A (2.75 μg/g creatinine /3.00 μg/L, GMc); 4-Nonylphenol (15.92 μg/g creatinine/17.40 μg/L, GM); Triclosan (3.55 μg/g creatinine /3.77 μg/L, GM) | 162 193⇓~195 160 |
| Fingernail | China | Triclosan (5.67 μg/kg, mean/2.0~760.7 μg/kg); Triclocarban (41.50 μg/kg, mean/4.1~1926.5 μg/kg) | 162 |
| Toenail | China | Triclosan (13.57 μg/kg, mean); Triclocarban (84.66 μg/kg, mean) | 162 |
| Skin | China | Galaxolide fragrance (HHCB) (7.8 μg/(kg·d);, median); Siloxanes ( (3.69 mg/d, median); Synthetic musks (3.38 mg/d, median) | 196,197 |
| Faeces | China | Sulfamethoxazole (45.4 μg/kg, max); Trimethoprim (38.33 μg/kg, max); Sulfadimethoxine (16.78 μg/kg, max); Tetracycline (38.65 μg/kg, max); Erythromycin (36.79 μg/kg, max); Amoxicillin (43.72 μg/kg, max) | 167 |
a,the concentration without special annotation means the range;b,lw=lipid weight;c,GM=geometric mean。 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
(王丹丹, 张婧, 杨桂朋, 李锋民. 环境科学研究., 2018, 31 (12): 2013.)
|
| [12] |
(邹艳敏, 吴向阳, 仰榴青. 环境监测管理与技术, 2010, 22(6): 14.).
|
| [13] |
(贾瑷, 胡建英, 孙建仙, 施嘉琛. 化学进展, 2009, 21(2-3):389.).
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
(张智博, 段艳平, 沈嘉豪, 俞文韬, 罗鹏程, 涂耀仁, 高峻, 环境科学, 2022, 43(01):349.).
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
(王博欣, 李炳华, 张大胜, 张世伟, 霍丽涛, 王靖. 南水北调与水利科技, 2020, 18 (6): 138.).
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
(尹承南, 谢培, 焦萌, 郭宝元, 于彩虹. 环境化学, 2021, 40(9): 2820.).
|
| [58] |
|
| [59] |
(马健生, 王卓, 张泽宇, 刘强, 李丽君. 岩矿测试, 2021, 40 (6): 944.).
|
| [60] |
|
| [61] |
(张盼伟. 中国水利水电科学研究院博士论文, 2018.).
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
(谢正鑫, 陆光华, 孙丽莎, 袁陆妗, 丁剑楠. 水资源保护, 2013, 29(4): 5.).
|
| [71] |
(张照荷, 陈典, 赵微, 袁国礼, 李俊, 焦杏春. 岩矿测试, 2023, 42(4): 649.).
|
| [72] |
(李伟明, 鲍艳宇, 周启星. 应用生态学报, 2012, 23(8): 2300.).
|
| [73] |
(高雪泉, 大连理工大学硕士论文, 2016.)
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
(郑丽英. 广州化工, 2010, 38(12):182.).
|
| [79] |
|
| [80] |
|
| [81] |
(黎展毅, 广东工业大学博士论文, 2018.)
|
| [82] |
(安继斌, 兰州理工硕士论文, 2011.).
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
(南彦斌, 曾立云, 马娟, 刘宏, 陈永志, 水处理技术, 2017, 43 (5): 16.).
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
(杨雪莹, 宿承璘, 高子晴, 大连工业大学学报, 2019, 38 (6):408.).
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
(高思越. 浙江大学硕士论文, 2021.).
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
(唐丽, 孙秀发, 张军, 中国药理学与毒理学杂志, 2002, 16 (1): 70.).
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
(刘娣, 浙江大学博士论文, 2010.).
|
| [149] |
(鲍陈燕, 顾国平, 章明奎, 土壤通报., 2016, 47 (1): 164.).
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
(胡洪营, 王超, 郭美婷, 生态环境, 2005, 14 (6): 947.).
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
(张品, 张晶, 石莹, 邵兵, 卫生研究, 2015, 44 (2): 294.).
|
| [183] |
(梁高锋, 王珺, 周静, 张晓岚, 盛国英, 傅家谟, 环境化学, 2010, 29 (1): 113.).
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [194] |
|
| [195] |
|
| [196] |
|
| [197] |
|
/
| 〈 |
|
〉 |